- HOME
- Laboratories
- Experimental Chemotherapy
- Projects
Research introduction and trait
Cancer treatments have dramatically improved during past 2 decades, however, we are still facing problems to surmount in prevention and therapy of cancers. Recent advances in molecular-targets of cancers have provided the rationale for the molecular-targeted therapy to treat cancer, and often showed market tumor shrinkage. The aim of this research division is to identify possible targets, to clarify the function of molecular targets by analyzing cancer patients derived specimens including pre-treatment and treatment refractory tumors, and ultimately to develop an effective molecular-targeted therapy of cancer. For this purpose, we are investigating the molecular mechanisms of anti-cancer drug resistance, apoptosis resistance, and tumor metastasis. In addition, we are investigating the nature of cancer stem cells.
Projects
- Resistance mechanisms to molecular targeted therapy, and therapeutic strategies to overcome the resistance
- Targeting tumor cell-platelet interactions for cancer therapy
- Targeting cancer stem cells and their regulation
- Molecular mechanisms of survival signaling pathways
Resistance to molecular targeted therapy, and therapeutic strategies to overcome the resistance
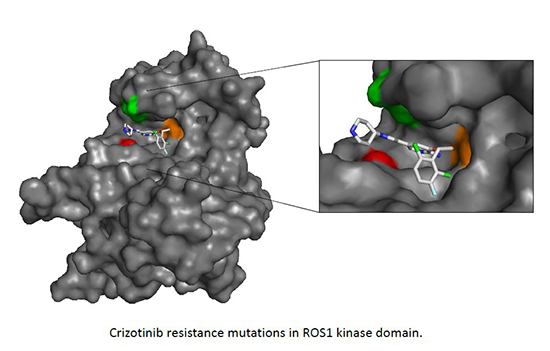
One of the goal of our research is to identify the molecular mechanisms of the drug resistance against molecular targeted therapy in cancer, and find the therapeutic strategies to overcome the resistance. Especially, we are focusing on lung cancer. Lung cancer is the leading cause of cancer death in Japan. According to the development of sequencing technologies and the enormous effort of the research, lung cancer is now defined by driver oncogene mutations, such as EGFR or KRAS active mutation, ALK, ROS1, or RET gene rearrangement, and molecular target therapy to each oncogenic mutated (altered) protein such as EGFR or ALK are used in clinic. For example, for the treatment of ALK rearranged lung cancer, currently ALK tyrosine kinase inhibitors, crizotinib, alectinib or ceritinib (only in US and EU) are used in clinic based on the remarkable responses in clinical trials. Although the molecular targeting drugs often shows drastic responses, tumor inevitably relapses due to the acquired resistance. To understand the resistance mechanisms to molecular targeted drugs, mainly tyrosine kinase inhibitors, in ALK, ROS1, RET rearranged NSCLC, we are establishing the resistant cells in vitro and in vivo and examine the mechanisms of resistance. In addition, by collaborating with the physician in our cancer institute hospital, we also examined the resistant mechanisms using the samples derived from molecular targeting drug refractory patients under approved IRB with signed Informed consent.
As one of the example, we have identified two novel ALK mutations (V1180L and I1171T) arising after alectinib exposure that are sensitive to other next-generation ALK inhibitors (Ceritinib). In addition, we developed a comprehensive model of acquired resistance to ROS1 inhibitors in NSCLC with ROS1 rearrangement and identified cabozantinib or DS-6051b (in collaboration) as a therapeutic strategy to overcome the resistance.
Recent Publications
- Ono F, Chiba S, Isaka Y, Matsumoto S, Ma B, Katayama R, Araki M, Okuno Y. Improvement in predicting drug sensitivity changes associated with protein mutations using a molecular dynamics based alchemical mutation method. Sci Rep. 2020 Feb 7;10(1):2161.
- Arai S, Takeuchi S, Fukuda K, Taniguchi H, Nishiyama A, Tanimoto A, Satouchi M, Yamashita K, Ohtsubo K, Nanjo S, Kumagai T, Katayama R, Nishio M, Zheng MM, Wu YL, Nishihara H, Yamamoto T, Nakada M, Yano S. Osimertinib Overcomes Alectinib Resistance Caused by Amphiregulin in a Leptomeningeal Carcinomatosis Model of ALK-Rearranged Lung Cancer. J Thorac Oncol. 2020 Jan 21. pii: S1556-0864(20)30022-8.
- Yanagitani N, Uchibori K, Koike S, Tsukahara M, Kitazono S, Yoshizawa T, Horiike A, Ohyanagi F, Tambo Y, Nishikawa S, Fujita N, *Katayama R, *Nishio M. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci. 2020 Mar;111(3):932-939.
- Takahashi K, Seto Y, Okada K, Uematsu S, Uchibori K, Tsukahara M, Oh-Hara T, Fujita N, Yanagitani N, Nishio M, Okubo K, *Katayama R. Overcoming resistance by ALK compound mutation (I1171S + G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer. Thorac Cancer. 2020 Mar;11(3):581-587.
- *Katayama R, Gong B, Togashi N, Miyamoto M, Kiga M, Iwasaki S, Kamai Y, Tominaga Y, Takeda Y, Kagoshima Y, Shimizu Y, Seto Y, Oh-hara T, Koike S, Nakao N, Hanzawa H, Watanabe K, Yoda S, Yanagitani N, Hata A, Shaw AT, Nishio M, Fujita N, Isoyama T. The new-generation selective ROS1/NTRK Inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nature Commun. 2019; Aug 9;10(1):3604.
- Fukuda K, Takeuchi S, Arai S, Katayama R, Nanjo S, Tanimoto A, Nishiyama A, Nakagawa T, Taniguchi H, Suzuki T, Yamada T, Nishihara H, Ninomiya H, Ishikawa Y, Baba S, Takeuchi K, Horiike A, Yanagitani N, Nishio M, Yano S. Epithelial-to-mesenchymal transition is a mechanism of ALK inhibitor resistance in lung cancer independent of ALK mutation status. Cancer Res. 2019; 79:1658-1670.
- Okada K, Araki M, Sakashita T, Ma B., Kanada R, Yanagitani N, Horiike A, Koike S, Oh-Hara T, Watanabe K, Tamai K, Maemondo M, Nishio M, Ishikawa T, Okuno Y, Fujita N, *Katayama R Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine, 2019; 41:105-119.
- Gong B, Oh-Hara T, Fujita N, *Katayama R. 3D culture system containing gellan gum restores oncogene dependence in ROS1 rearrangements non-small cell lung cancer. Biochem Biophys Res Commun. 2018 Jun 22;501(2):527-533.
- Uchibori K, Inase N, Nishio M, Fujita N, *Katayama R. Identification of Mutation Accumulation as Resistance Mechanism Emerging in First-Line Osimertinib Treatment. J Thorac Oncol. 2018 Jul;13(7):915-925.
- Ariyasu R, Nishikawa S, Uchibori K, Oh-Hara T, Yoshizawa T, Dotsu Y, Koyama J, Saiki M, Sonoda T, Kitazono S, Yanagitani N, Horiike A, Inase N, Kasahara K, Nishio M, *Katayama R. High ratio of T790M to EGFR activating mutations correlate with the osimertinib response in non-small-cell lung cancer. Lung Cancer. 2018 Mar;117:1-6.
- *Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci. 2018 Mar;109(3):572-580. Review.
- Fuse MJ, Okada K, Oh-hara T, Ogura H, Fujita N, *Katayama R. Mechanisms of resistance to NTRK inhibitors and therapeutic strategies in NTRK1-rearranged cancers. Mol Cancer Ther. 2017, October 1;16 (10):2130-2143.
- Ogura H, Nagatake-Kobayashi Y. Adachi J, Tomonaga T, Fujita N, *Katayama R. TKI-addicted ROS1-rearranged cells are destined to survival or death by the intensity of ROS1 kinase activity. Sci Rep. 2017, July 17; 7: 5519
- Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, Fujita N, *Katayama R. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nature Commun., 2017 Mar 13;8:14768.
- *Katayama R. Therapeutic strategies and mechanisms of drug resistance in anaplastic lymphoma kinase (ALK)-rearranged lung cancer. Pharmacol Ther. 2017 Sep;177:1-8. Review.
- Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K, Singh M, Chin E, Parks M, Lee D, DiCecca RH, Lockerman E, Huynh T, Logan J, Ritterhouse LL, Le LP, Muniappan A, Digumarthy S, Channick C, Keyes C, Getz G, Dias-Santagata D, Heist RS, Lennerz J, Sequist LV, Benes CH, Iafrate AJ, Mino-Kenudson M, Engelman JA, *Shaw AT. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6(10):1118-1133.
- Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, Burke BJ, Deng YL, Liu W, Dardaei L, Frias RL, Schultz KR, Logan J, James LP, Smeal T, Timofeevski S, Katayama R, Iafrate AJ, Le L, McTigue M, Getz G, Johnson TW, *Engelman JA. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med. 2016 Jan 7;374(1):54-61.
- *Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A, Sakata S, Tambo Y, Kitazono S, Sato S, Koike S, John Iafrate A, Mino-Kenudson M, Ishikawa Y, Shaw AT, Engelman JA, Takeuchi K, *Nishio M, *Fujita N. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine, 2016 Jan; 3: 54–66.
- *Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015 May 15;21(10):2227-35. Review.
- Katayama R, Kobayashi Y, Friboulet L, Lockerman EL, Koike S, Shaw AT, Engelman JA, *Fujita N. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015 Jan 1;21(1):166-74.
- Katayama R, Friboulet L, Koike S, Lockerman EL, Khan TM, Gainor JF, Iafrate AJ, Takeuchi K, Taiji M, Okuno Y, Fujita N, *Engelman JA, *Shaw AT. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res.2014 Nov 15;20(22):5686-96.
- Friboulet L†, Li N†, Katayama R†, Lee CC, Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S, Pferdekamper AC, Li J, Kasibhatla S, Sun F, Sun X, Hua S, McNamara P, Mahmood S, Lockerman EL, Fujita N, Nishio M, Harris JL, Shaw AT, *Engelman JA. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014 Jun;4(6):662-73. †: co-first authors
Targeting tumor cell-platelet interactions for cancer therapy
Our goal of this project is to unveil the molecular mechanism of platelet-mediated tumor malignancy and to develop the molecular targeted cancer therapy which blocks tumor cell-platelet interactions. Tumor cell-induced platelet aggregation facilitates hematogenous metastasis by promoting tumor embolization, preventing immunological assaults and shear stress, and the platelet-releasing growth factors which support tumor growth and invasion. In 2003, we identified podoplanin (PDPN), also known as Aggrus, as a platelet activation-inducing molecule expressing on the surface of highly-metastatic cancer cells (J Biol Chem 2003) . CHO cells stably overexpressing PDPN exhibit high platelet aggregation-inducing ability in vitro and frequently form the pulmonary metastasis in experimental pulmonary metastasis models (Am J Pathol 2007) . Although PDPN has been well known as a lymphatic endothelial marker molecule, its function was unknown before our findings. Overexpression of PDPN is observed in wide range of tumor types, including lung squamous cell carcinoma, bladder cancer, osteosarcoma, malignant pleural mesothelioma, glioblastoma, and seminoma (Oncogene 2004; Tumour Biol 2005; Int J Cancer 2014) .

Recently, we reported that PDPN-mediated platelet aggregation induces the release of TGF-β, PDGF, and EGF from activated platelets, resulting in cancer cells EMT (Sci Rep 2017) and enhancing proliferation of lung squamous cell carcinoma and osteosarcoma cells (Cancer Sci 2014; Sci Rep 2017). These findings indicate that tumor cell-induced platelet activation contributes not only to tumor metastasis but also to tumor malignancy. We are currently conducting further research to elucidate the detailed mechanisms of cancer malignancy mediated by platelets.
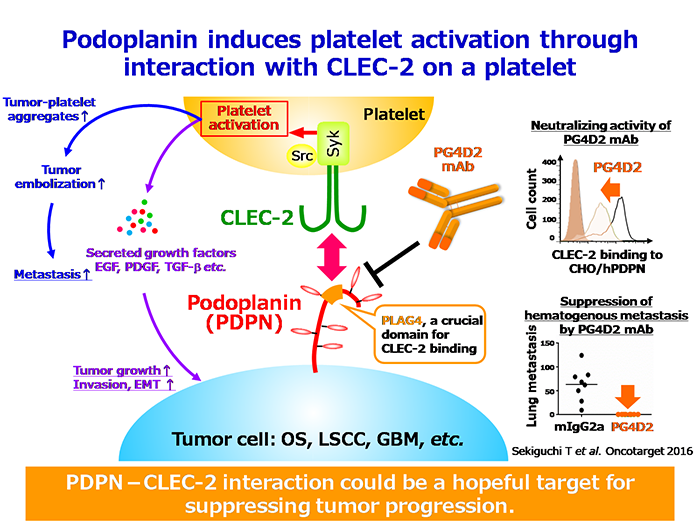
PDPN-induced platelet activation and metastasis formation are mediated by the interaction with its platelet receptor, C-type lectin-like receptor 2 (CLEC-2). We identified that PDPN contains a highly conserved sequence beyond species called “PLAG domain” (PLAG1-3), and these regions are important for its binding to CLEC-2 (J Biol Chem 2004) .
We recently identified “PLAG4 domain”, a new functional region essential for the PDPN-CLEC-2 interaction, and successfully established anti-human PDPN neutralizing antibodies, PG4D1 and PG4D2, which specifically recognize the human PLAG4 domain (Oncotarget 2016) . Moreover, we also established an anti-monkey PDPN neutralizing antibody, 2F7 mAb, which recognizes the monkey PLAG4 domain, and confirmed that a single dose intravenous administration of 2F7 mAb exhibits no acute toxicity to cynomolgus monkeys in the histological and hematological examinations (Oncotarget 2018) . Based on the favorable results of the safety test with monkeys, we have developed a humanized anti-PDPN neutralizing antibodies in collaboration with a company, and are currently preparing for preclinical studies.
Recent publications:
- P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. *Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A, Sakata S, Tambo Y, Kitazono S, Sato S, Koike S, John Iafrate A, Mino-Kenudson M, Ishikawa Y, Shaw AT, Engelman JA, Takeuchi K, *Nishio M, *Fujita N.
EBioMedicine, 2016 Jan; 3: 54–66. - Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells.
Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR, Yashiro M, Hirakawa K, Aburatani H, Fujita N, Miyazono K.
Oncogene, 30: 1693-1705, 2011. - AP-1-dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells.
Misawa A, Katayama R, Koike S, Tomida A, Watanabe T, *Fujita N.
Oncol. Res., 2010;19(1):23-33 - Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export.
Katayama R, Koike S, Sato S, Sugimoto Y, Tsuruo T, *Fujita N.
Cancer Sci. 2009 Nov;100(11):2060-8.
Targeting cancer stem cells and their regulation
During the cancer chemotherapy, the "Relapse" makes it difficult to achieve complete cure of cancer. It is thought that present anti-cancer drugs could not kill small population of cancer cells. The cells were called "cancer stem cells". They were known to possess stem cell like properties. Therefore, we are now trying to clarify the properties of the cancer stem cells and to find the new strategies to kill cancer stem cells.

The role of cancer stem cells in re-generation.
Because cancer stem cells (shown in red) show resistance to anti-cancer drugs, cancer stem cells can survive under conventional cancer chemotherapy. Therefore, re-generation is rapidly occurred after drug withdrawal.
Recent publications:
- P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. *Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A, Sakata S, Tambo Y, Kitazono S, Sato S, Koike S, John Iafrate A, Mino-Kenudson M, Ishikawa Y, Shaw AT, Engelman JA, Takeuchi K, *Nishio M, *Fujita N.
EBioMedicine, 2016 Jan; 3: 54–66. - Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells.
Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR, Yashiro M, Hirakawa K, Aburatani H, Fujita N, Miyazono K.
Oncogene, 30: 1693-1705, 2011. - AP-1-dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells.
Misawa A, Katayama R, Koike S, Tomida A, Watanabe T, Fujita N.
Oncol. Res., 2010;19(1):23-33 - Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells.
Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR, Yashiro M, Hirakawa K, Aburatani H, Fujita N, Miyazono K.
Oncogene, 2011 Apr 7;30(14):1693-705
- Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export.
Katayama R, Koike S, Sato S, Sugimoto Y, Tsuruo T, Fujita N.
Cancer Sci. 2009 Nov;100(11):2060-8.





