3.How do cells induce an abrupt separation of chromosomes? 4.Mechanisms of chromosome instability in cancer cells
1.How chromosomes are assembled in mitosis?
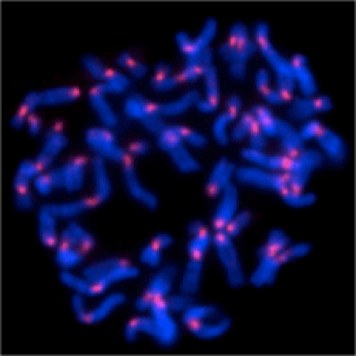
The condensation of chromatin fiber initiates in prophase, and it progressively condense to form chromosomes prior to its segregation. In parallel, sister chromatids dissolve from each other and pairs of chromosome ´arms´ become discernible. Condensin complexes, which are thought to be involved in chromosome condensation, gain association with chromosomes. By contrast, cohesin complexes, which hold sister chromatids, are removed from chromatin during chromosome assembly. We are studying how the dynamic metabolism of these chromosomal proteins is controlled to assemble chromosomes in mitosis.
2.How kinetochore-microtubule attachment is controlled?
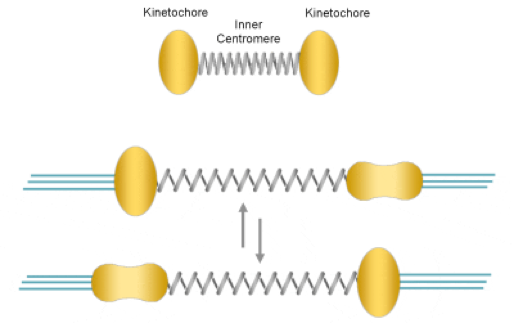
Accurate separation of sister chromatids is possible only when all the chromosomes achieve "bi-orientation", a condition where sister kinetochores of each chromosome are attached to microtubule bundles emanating from opposite spindle poles. Because microtubule attachment is principally a trial-and-error process, incorrect attachments can happen. If anaphase occurs in the presence of erroneous attachments chromosomes can be separated unequally, giving rise to the generation of aneuploid cells. In order to prevent such pathological situation, cells are equipped with at least two machineries, namely the correction mechanism and the spindle assembly checkpoint.
3.How do cells induce an abrupt separation of chromosomes?
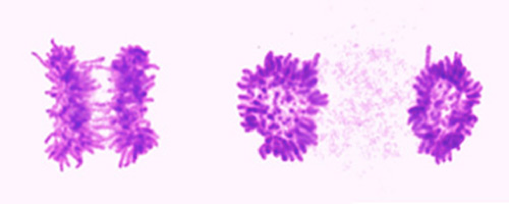
Separation of sister chromatids has long been recognized as a drastic and irreversible event in the cell cycle. A fascinate feature of this process in mammalian cells is that the separation occurs in a highly synchronous manner at the given moment: all sister pairs separate at the same time. This abruptness of anaphase onset is phenomenologically well known, but we are far from understanding it at the mechanistic levels. I am very interested in understanding how do cells achieve this remarkable feat, and therefore we aim to characterize morphological and biochemical changes during the metaphase-to-anaphase transition, in high time and space resolution.
4.Mechanisms of chromosome instability in cancer cells
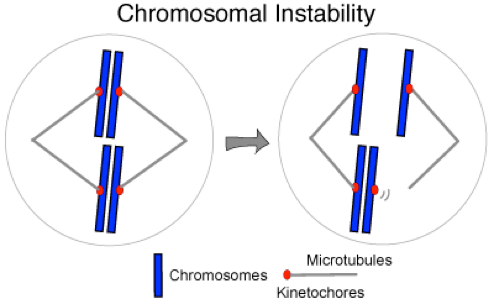
Many malignant tumors are aneuploid, and reveal high rates of chromosome segregation defects as cells devide. This genetic heterogeneity is thought to contribute to malignant phenotypes. Studies into the basic mechanisms of chromosome segregation have revealed many pathways that might cause chromosomal instability. Based on our knowledge about the physiological regulation of mitosis, we are now ready to characterize chromosome dynamics in cancer cells, in order to investigate the ´lesion´ in chromosome segregation machinery. These approaches should lead to improved treatments for cancer.