The Outline of Research in Hara’s Lab
Due to improvements in healthcare and lifestyle over the past decades, human life expectancy has increased dramatically and people are now living longer, especially in developed countries. Ironically, however, extended lifespan has resulted in a startling rise in the incidence of cancer. To have a meaningful impact on the continued healthcare and well-being of this aging population, there is an urgent need for increased understanding of the molecular mechanisms underlying aging-associated cancer. We think that cellular senescence plays key roles linking aging and cancer.
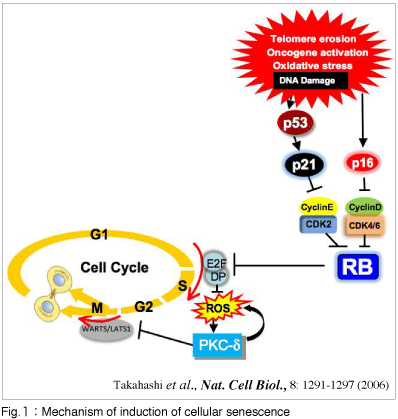
Cellular senescence is the state of essentially irreversible cell cycle arrest that can be induced by a variety of potentially oncogenic stimuli, such as telomere erosion, oxidative stress or activation of certain oncogenes and is considered to act as an important tumor suppression pathway (Fig.1).
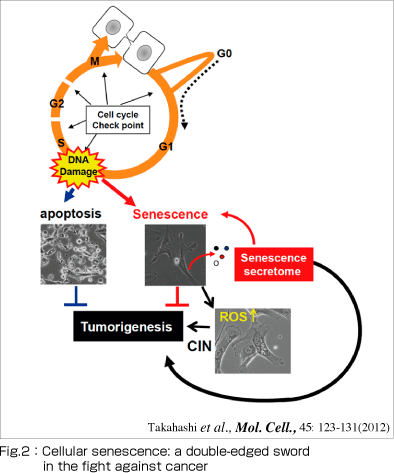
However, emerging evidence indicates that senescent cells may also promote deleterious side-effects including chronic inflammation and cancer promotion (Fig.2).
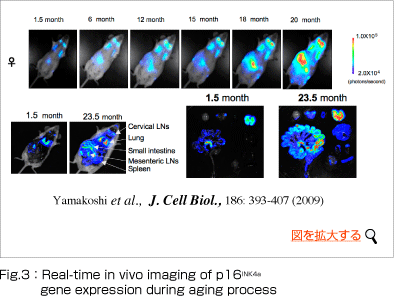
It is therefore quite possible that accumulation of senescent cells in vivo may contribute to age-related increases in cancer (Fig.3).
In our laboratory, we are studying on the molecular mechanisms associated with these two faces of cellular senescence (Fig. 2), aiming to understand the molecular link between aging and cancer. We believe that a better understanding of the molecular mechanisms involved will lead to new strategies for the prevention of aging-associated cancer.





