- HOME
- Outstanding Progress
Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer
Hayato Mizuta1,2†, Koutaroh Okada1,3†, Mitsugu Araki4, Jun Adachi5, Ai Takemoto1, Justyna Kutkowska1, Kohei Maruyama1,3, Noriko Yanagitani6, Tomoko Oh-hara1, Kana Watanabe7, Keiichi Tamai8, Luc Friboulet9, Kazuhiro Katayama10, Biao Ma11, Yoko Sasakura11, Yukari Sagae4, Mutsuko Kukimoto-Niino12, Mikako Shirouzu12, Satoshi Takagi1, Siro Simizu2, Makoto Nishio6, Yasushi Okuno4, Naoya Fujita1, Ryohei Katayama1,3*
Nature Communications, 12, 1261 (2021)
Gilteritinib overcomes lorlatinib resistance in ALK -rearranged cancer | Nature Communications
- Division of Experimental Chemotherapy, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, Tokyo, Japan.
- Department of Applied Chemistry, Faculty of Science and Technology, Keio University, Kanagawa, Japan.
- Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Tokyo, Japan.
- Graduate School of Medicine, Kyoto University, Kyoto, JAPAN
- Laboratory of Proteomics for Drug Discovery, Laboratory of Clinical and Analytical Chemistry, Center for Drug Design Research, National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan.
- Department of Thoracic Medical Oncology, The Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan.
- Department of Respiratory Medicine, Miyagi Cancer Center, Miyagi, Japan.
- Division of Cancer Stem Cell, Miyagi Cancer Center Research Institute, Miyagi, Japan.
- INSERM U981, Gustave Roussy Cancer Campus, Université Paris Saclay, Villejuif, France.
- Laboratory of Molecular Targeted Therapeutics, School of Pharmacy, Nihon University, Chiba, Japan
- Research and Development Group for In Silico Drug Discovery, Center for Cluster Development and Coordination (CCD), Foundation for Biomedical Research and Innovation at Kobe (FBRI), Kobe, Japan.
- RIKEN Center for Biosystems Dynamics Research, Kanagawa, Japan
†These authors contributed equally to this work.
ALK-rearranged NSCLCs account for 3%–5% of lung adenocarcinoma. These ALK fusion proteins can induce the constitutive activation of the ALK-tyrosine kinase and its downstream pathways. To date, 5 ALK-tyrosine kinase inhibitors (TKIs) are approved and showed a clinical efficacy in ALK-positive NSCLC patients. However, cancer cells inevitably acquire drug resistance, resulting in tumor recurrence. One of the resistant mechanisms is associated with mutations in ALK kinase region. While some of them are overcome by lorlatinib, the third generation ALK-TKI, compound mutations such as I1171N + F1174I and I1171N + L1198H are resistant to all clinically approved ALK-TKIs. Thus, the identification of a novel therapeutic inhibitor was strongly desired.
In this study, we found that gilteritinib, a TKI approved for treating relapsed or refractory FLT3 positive acute myeloid leukemia (AML), can overcome these ALK-TKI resistant compound mutations. Then, we investigated whether gilteritinib has inhibitory effects on other single or compound mutants of ALK. Using cell viability assays with EML4-ALK (WT or mutants) overexpressed Ba/F3 cells and western blot analysis, the marked antitumor effects of gilteritinib against cells carrying various single and compound mutations were observed (Figure 1a). From the invitro kinase assay, gilteritinib directly inhibited ALK tyrosine kinase in an ATP competitive manner, and gilteritinib fitted in ATP binding pocket of ALK (Figure 1b). These results were also confirmed using ALK-rearranged NSCLC patients-derived cells in vitro and in vivo (Figure 1c and 1d).
Our study represents the first report of the effectiveness of gilteritinib against ALK-TKI resistance ALK rearranged cancer, and these findings might provide beneficial information for the identification of additional indications for gilteritinib.
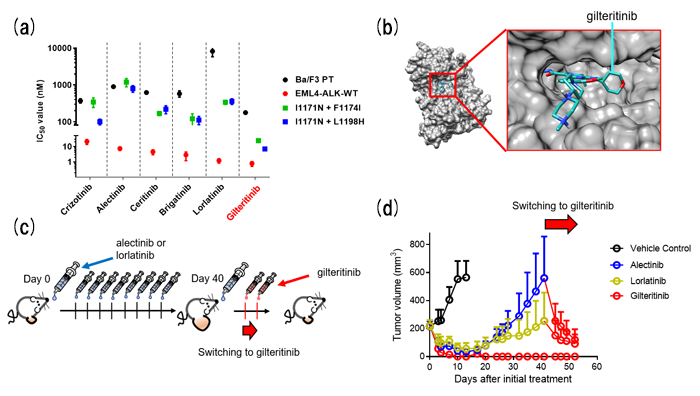
Figure. Gilteritinib overcomes lorlatinib resistance.
(a) IC50 calculated from the viability analysis of parental Ba/F3 cells or carrying indicated EML4-ALK. (b) The ALK-gilteritinib complex structure predicted by the molecular docking and molecular dynamic simulation. (c) Schematic of in vivo study. (d) EML4-ALK I1171N + F1174I overexpressed patient derived cells were subcutaneously transplanted and treated with indicated ALK-TKIs





