- HOME
- Laboratories
- Molecular Biotherapy
- Projects
Research introduction and trait
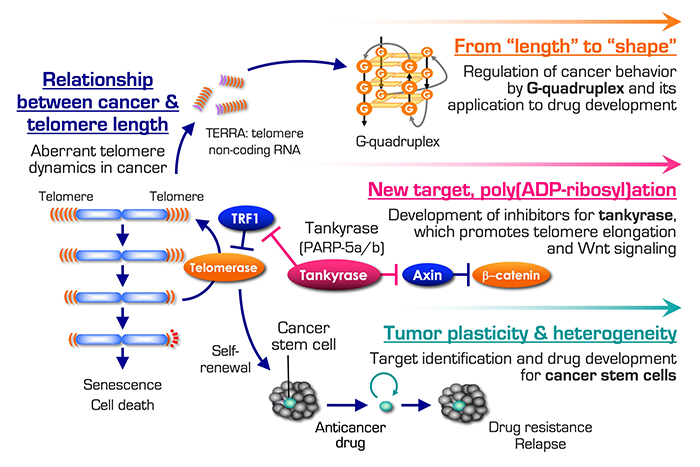
We have been concentrating our efforts to develop new cancer molecular medicine. At the starting point, we focused on telomere maintenance system and developed various telomerase inhibitors that block infinite cancer cell growth. This fundamental achievement and related basic studies have expanded the opportunities to discover innovative drug seeds. First, we are studying G-quadruplexes (G4s), atypical higher-order structures formed at telomeres and other guanine-rich genomic loci. Our newly synthesized G4-stabilizing agents inhibit the growth of glioblastoma and its cancer stem cells in vivo. Second, we are developing specific inhibitors of tankyrase, a poly(ADP-ribose) polymerase that facilitates telomere elongation. Tankyrase is also a positive regulator of Wnt signaling, and we have confirmed that our tankyrase inhibitors block the growth of Wnt-driven colorectal cancer cells in vivo.
Projects
- Telomeres and cancer cell immortality
- G-quadruplex as an anticancer therapeutic target
- Tankyrase, a poly(ADP-ribose) polymerase, and its inhibitors
- Cancer stem cells as therapeutic targets
Telomeres and cancer cell immortality
Telomere maintenance and cell immortality by telomerase
To proliferate, cells need to replicate their DNA. However, classical replication machinery cannot completely replicate the very ends of linear chromosomes. Thus, telomeres, specialized structures that protect eukaryotic chromosome termini, gradually shorten after each cell cycle. When telomeres reach the limit of shortening, the cells cannot divide any further. This phenomenon, called replicative senescence ("aging" of a cell), is one of the systems that prevent carcinogenesis. In most cancer cells, the telomere-synthesizing enzyme, telomerase, stably maintains telomeres. Accordingly, cancer cells have the ability to divide infinitely.
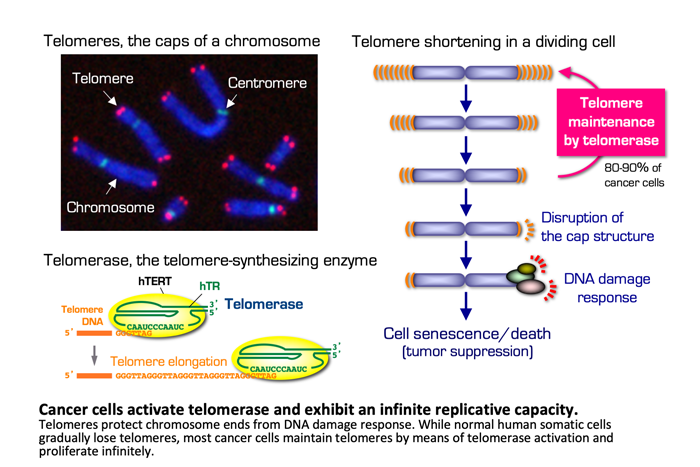
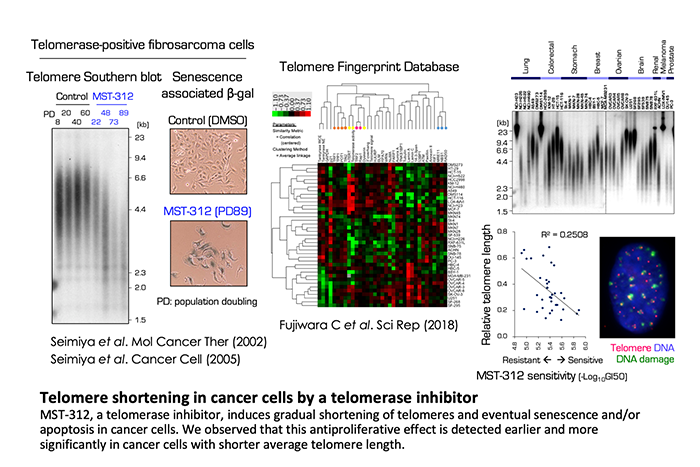
We have developed a series of telomerase inhibitors (e.g., MST-312, 295, 199) that block the unlimited growth of cancer cells. We also found that each cancer cell line exhibits a differential telomere status, in terms of its DNA length and composition of the binding proteins. We confirmed that cancer cells with shorter telomeres tend to be more sensitive to the deleterious effects of telomerase inhibitors.
Telomere paradox in cancer
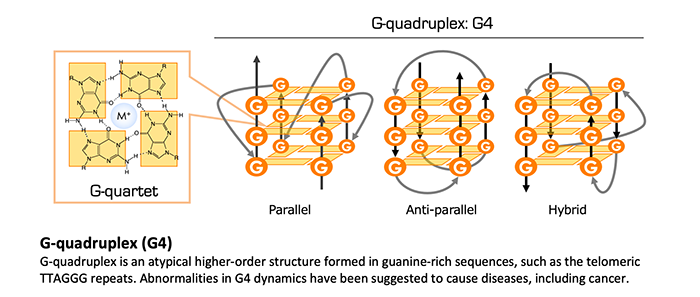
Because telomeric DNA consists of 5’-TTAGGG-3’ repetitive sequence and does not code any proteins, it had been long believed that telomeres are transcriptionally silent. However, telomeric DNA is actually transcribed to a non-coding RNA called TERRA (telomeric repeat-containing RNA), which plays roles for various events, including telomere protection, heterochromatin formation and telomerase inhibition. Interestingly, telomeric sequence can form a higher-order nucleic acid structure called G-quadruplex (G4). While G4 was originally described in vitro, it is detected in living cells and has become one of the hot topics in the area of genome biology. It has been postulated that abnormalities in G4 dynamics can lead to neurodegenerative diseases and cancer. This suggests that G4 could be utilized as a diagnostic biomarker and a therapeutic target of such diseases.
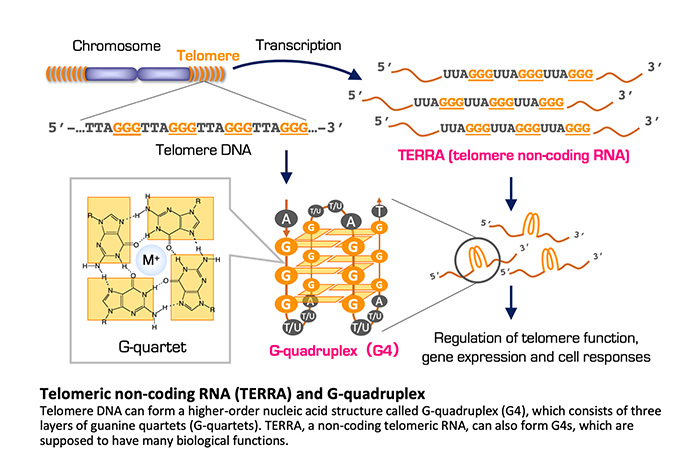
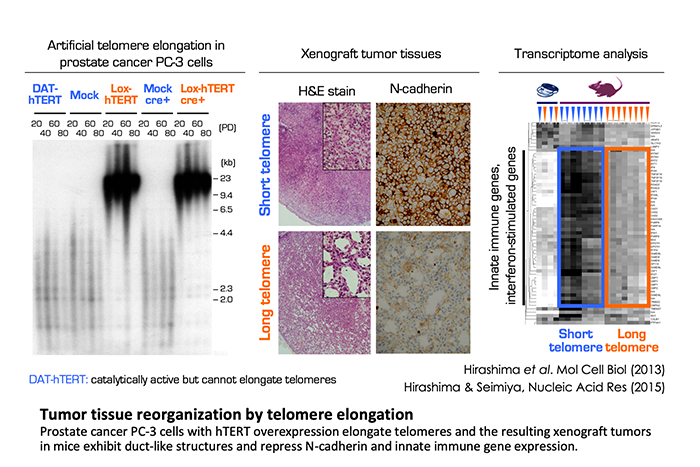
We are interested in the fact that TERRA can form G4, and are investigating the possibility that TERRA-G4 is involved in pathological regulation of cancer behavior. At the starting point, we focused on the telomere length in cancer cells. Given that telomere length is a mitotic clock that counts the number of cell division, it is assumed that longer telomeres would be more advantageous for repetitive cell division. Intriguingly, however, cancer cells often maintain their telomeres shorter than normal cells. This paradoxical phenomenon has prompted us to examine the functional significance of telomere length in cancer. When telomeres are artificially elongated in human prostate, stomach and breast cancer cell lines, the resulting xenograft tumors in mice exhibit altered gene expression patterns and tissue reorganization, as compared to the tumors derived from the parental cancer cells. The downregulated genes in the telomere-elongated tumors include those involved in innate immune and interferon responses, which are overexpressed in various cancers. Importantly, expression for these genes are repressed by TERRA G4 and other G4-forming oligonucleotides. These observations suggest that G4 regulates gene expression in a genome-wide manner and cancer cells may maintain their telomeres short to allow induction of innate immune and interferon-stimulated genes for their malignant phenotype.
G-quadruplex as an anticancer therapeutic target
What is G-quadruplex?
The most typical DNA structure is the right winding, Watson-Crick type double helix, which assures the template-dependent and semiconservative DNA replication and contributes to precise propagation of genomic information. Meanwhile, guanine-rich strand of the telomeric repeat sequence can form an atypical higher-order structure called G-quadruplex (G4) under the physiological conditions with potassium or sodium ions. G4 are formed not only in DNA but also in RNA. Furthermore, G4 is formed at non-telomeric sequences, such as (GGGX1-7)4, in a genome-wide manner in intact cells. However, details of the regulation mechanism for G4 dynamics and the physiological significance of G4s remain elusive.
Anticancer effects of G4-stabilizing drugs (G4 ligands)
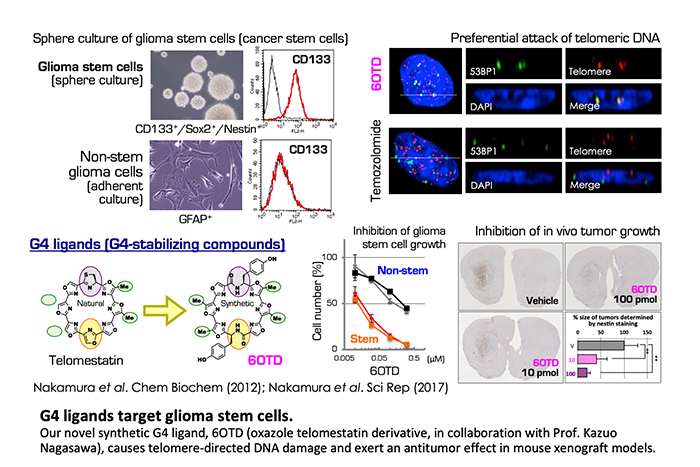
G4 dynamics can be controlled by chemical compounds called G4 ligands. These compounds specifically recognize G4s and stabilize them. We are studying cellular responses elicited by G4 ligands and the cell specificity for such responses. For example, we have reported that a natural G4 ligand, telomestatin, and its synthetic derivatives stabilize the telomeric G4 and cause prompt de-protection of chromosome ends. These ligands exhibit potent anticancer activities against glioma stem cells, which are the tumor-initiating cells of intractable glioblastoma. Mechanistically, glioma stem cells are found to be hypersensitive to the replication stress induced by G4 ligands. We have also identified other hypersensitive cancer cells and are investigating predictive biomarkers of the efficacies.
Tankyrase, a poly(ADP-ribose) polymerase, and its inhibitors
Poly(ADP-ribose) polymerases as anticancer targets
Poly(ADP-ribosyl)ation (PARylation), one of the most drastic post-translational modifications of proteins, is catalyzed by poly(ADP-ribose) polymerases (PARP). PARsylation regulates various biological events, including genomic stability, gene transcription, cell signaling and so forth. We still have many questions about this process, such as the molecular basis for functional specificity elicited from PAR chains. Recent reports including ours show that PARP inhibitors could be utilized as novel anticancer drugs. Especially, PARP-1 and PARP-2 inhibitors are effective to the homologous recombination-deficient (HRD) cancers, such those with the tumor suppressor BRCA1 or BRCA2 deficiency, and have been clinically approved in many countries. The potent and selective anticancer effects of PARP inhibitors on HRD cancer are derived from the synthetic lethality between the pharmacological effect of PARP inhibition and lack of an ability to repair DNA by homologous recombination in target cancer cells. Because BRCA1 and BRCA2 are tumor suppressor genes and often inactivated in cancer, it is impossible to directly pinpoint these targets. In that sense, it is of note that the synthetic lethal approach with PARP inhibitors can target the “absence” of tumor suppressors.
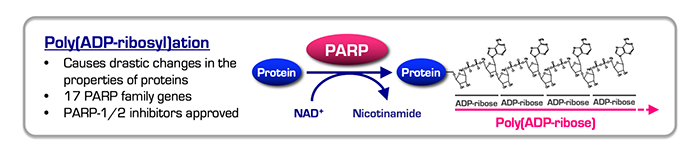
Tankyrase, a positive regulator of telomerase
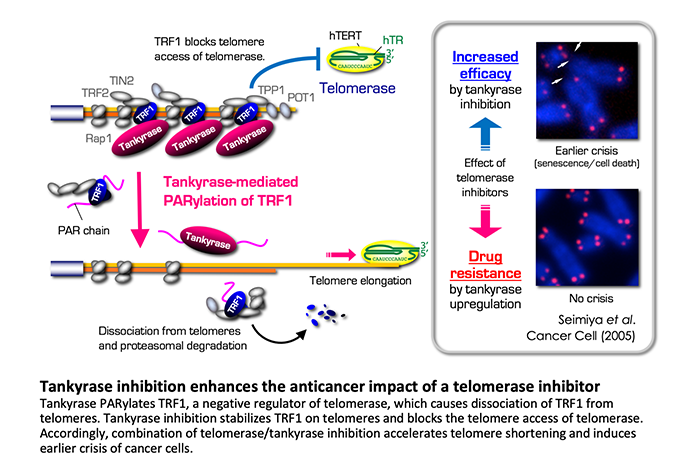
We focus on tankyrases (tankyrase-1/PARP-5a and tankyrase-2/PARP-5b), which enhance telomere elongation by telomerase in human cancer cells. Telomeres contain the protein complexes called shelterin. Among the shelterin components, the double-stranded telomere-binding protein TRF1 represses the telomere access of telomerase and works as a negative regulator for telomere length. Tankyrase PARylates TRF1 and this post-translational modification releases TRF1 from telomeres. As a result, telomerase can more easily access to telomeres and elongate the telomeric DNA. We have reported that tankyrase inhibition blocks the telomere access of telomerase and boosts the anticancer impact of a telomerase inhibitor.
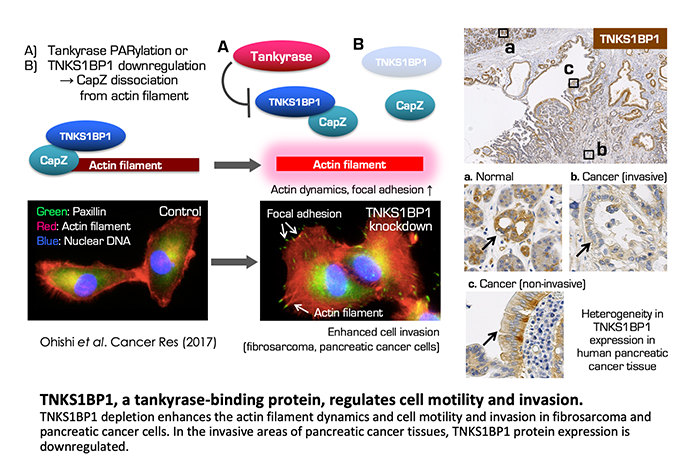
Meanwhile, we are also working on the multi-functionality of tankyrases. Tankyrase has five ankyrin repeat clusters (ARCs) as the multiple interaction platforms for various binding proteins, including TRF1. We identified several tankyrase-binding proteins, such as TNKS1BP1 and MERIT40, and found that these protein interactions with tankyrase regulate cell motility and DNA damage repair, respectively.
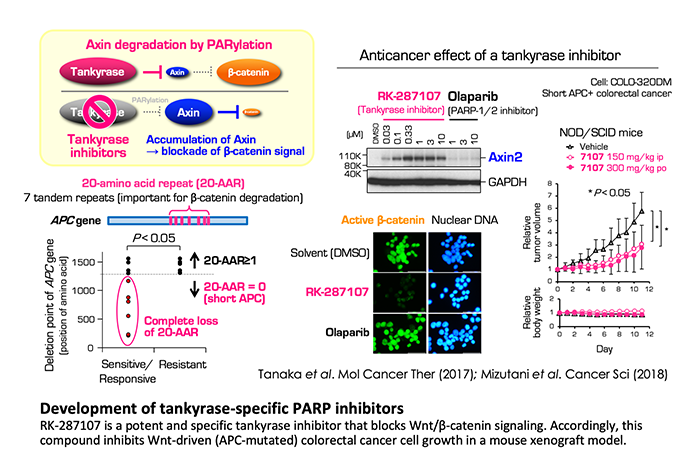
Development of tankyrase-specific PARP inhibitors
Tankyrase also enhances Wnt/β-catenin signaling, which is closely associated with carcinogenesis in various tissues, especially, in colorectal cancer. Historically, Wnt/β-catenin signaling pathway has been supposed to be less-druggable (hard to develop the molecularly targeted drugs). Tankyrase PARylates Axins, negative regulators of β-catenin, and induce proteasomal degradation of Axins. This results in accumulation of β-catenin and expression of the target genes. We have developed potent and specific tankyrase inhibitors, such as RK-287107 and RK-582, which induce accumulation of Axins and subsequent degradation of β-catenin. Accordingly, these inhibitors repress the growth of xenograft tumors of human colorectal cancer cells in a preclinical model. We also identified potential predictive biomarkers for tankyrase inhibitors, which could be applied for perspective precision medicine.
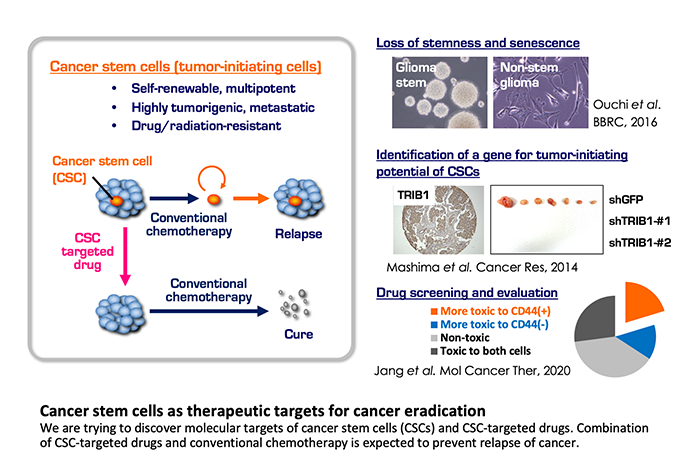
Cancer stem cells as therapeutic targets
Cancer heterogeneity and plasticity are derived from stochastic clonal evolution and cellular hierarchy, in the latter of which cancer stem cells (CSCs) reside on the top position. CSCs, which are self-renewable, multipotent, and highly tumorigenic, exhibit drug resistance and a metastatic potential. Therefore, CSCs are postulated as “a queen bee” that hinders cancer eradication. Molecular signaling pathways and microenvironmental niches that regulate survival, proliferation, and stemness of CSCs have been identified from various cancers. These factors would be promising targets for anticancer drug development. In our laboratory, we focus on glioma stem cells, prostate, colorectal and stomach CSCs, including those isolated from patient-derived cancer cells approved by the institutional review board and under informed consent from the patients. Employing functional genomics and comprehensive transcriptome analyses we are pursuing therapeutic targets of CSCs. As described above, we are also developing chemical compounds called G-quadruplex ligands, which have a preferential anti-proliferative effect on glioma stem cells.