- HOME
- Outstanding Progress
Division of Molecular Pharmacology contributes to the development of a new molecular-targeted drug
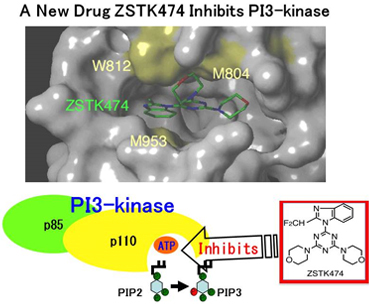 PI3-kinase (PI3K) is a lipid kinase playing a key role in the downstream signaling of receptor tyrosine kinases. Aberration of PI3K pathway promotes carcinogenesis and cancer progression. Therefore, PI3K is a promising target for cancer chemotherapy, and the development of PI3K inhibitors became an attractive hot area. In such background, Division of Molecular Pharmacology, DMP, (Chief, Takao Yamori, Ph.D.,) identified a novel PI3K inhibitor ZSTK474 in collaboration with Zenyaku Kogyo Co. Ltd (J Natl Cancer Inst 2006;98(8):545-56). That was the first report of a PI3K inhibitor, which has a potent in vivo antitumor activity. ZSTK474 is chemically stable, effective via oral administration and less toxic, which are favorable properties as a candidate of anticancer drug. Then, DMP started extensive investigation of molecular and biological properties of ZSTK474, and clarified its molecular mode of action, its specificity of enzyme inhibition, its efficacy in various animal cancer models and its antiangiogenic activity (see the footnote). In parallel, Zenyaku Kogyo completed requirements for entering clinical trial of ZSTK474, such as formulation optimization, pharmacokinetics and GLP. After the approval of IND by FDA, the phase I clinical trial of ZSTK474 finally started from January 2011 in United States (see Ref. 1). Moreover, DMP has recently shown, in collaboration with the team of Dr. Ryuichi Okayasu in National Institute of Radiation Science, that ZSTK474 has enhanced efficacy when combined with radiation therapy (see Ref. 2). This finding may push the clinical development of ZSTK474. The progress of clinical trial will be worthy of note.
PI3-kinase (PI3K) is a lipid kinase playing a key role in the downstream signaling of receptor tyrosine kinases. Aberration of PI3K pathway promotes carcinogenesis and cancer progression. Therefore, PI3K is a promising target for cancer chemotherapy, and the development of PI3K inhibitors became an attractive hot area. In such background, Division of Molecular Pharmacology, DMP, (Chief, Takao Yamori, Ph.D.,) identified a novel PI3K inhibitor ZSTK474 in collaboration with Zenyaku Kogyo Co. Ltd (J Natl Cancer Inst 2006;98(8):545-56). That was the first report of a PI3K inhibitor, which has a potent in vivo antitumor activity. ZSTK474 is chemically stable, effective via oral administration and less toxic, which are favorable properties as a candidate of anticancer drug. Then, DMP started extensive investigation of molecular and biological properties of ZSTK474, and clarified its molecular mode of action, its specificity of enzyme inhibition, its efficacy in various animal cancer models and its antiangiogenic activity (see the footnote). In parallel, Zenyaku Kogyo completed requirements for entering clinical trial of ZSTK474, such as formulation optimization, pharmacokinetics and GLP. After the approval of IND by FDA, the phase I clinical trial of ZSTK474 finally started from January 2011 in United States (see Ref. 1). Moreover, DMP has recently shown, in collaboration with the team of Dr. Ryuichi Okayasu in National Institute of Radiation Science, that ZSTK474 has enhanced efficacy when combined with radiation therapy (see Ref. 2). This finding may push the clinical development of ZSTK474. The progress of clinical trial will be worthy of note.
Ref. 1. A Safety Study of Oral ZSTK474 in Patients With Cancer
The safety of the daily oral administration of ZSTK474 for 21 days is under investigation. The detail is described in the following site:
http://www.clinicaltrial.gov/ct2/show/NCT01280487?term=ZSTK474&rcv_d=14![]()
Footnote: These studies were partly supported by a grant from the National Institute of Biomedical Innovation, Japan, and grants-in-aid of the Priority Area ‘Cancer’from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.





