- HOME
- Outstanding Progress
Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer
Ken Uchibori1,2, Naohiko Inase2, Mitsugu Araki3, Mayumi Kamada4, Shigeo Sato1, Yasushi Okuno3,4, Naoya Fujita1, Ryohei Katayama1*
NATURE COMMUNICATIONS, 8:14768, March 13, 2017
1 Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
2 Department of Respiratory Medicine, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113 - 8510, JAPAN
3 RIKEN Advanced Institute for Computational Science, 7-1-26 Minatojima-Minamimachi, Chuo-ku, Kobe, Hyogo 650-0047, JAPAN
4 Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaharacho, Sakyo-ku, Kyoto 606-8507, JAPAN
The lung cancer is a leading cause of the cancer death around the world. The non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer. The epidermal growth factor receptor (EGFR) mutation is a one of the driver oncogene observed in 30–40% or ~15% of NSCLC in Japan or North America and European countries, respectively. The EGFR tyrosine kinase inhibitor (EGFR-TKI) has shown the clinical benefit for EGFR mutation positive lung cancer, as their overall survival has been improved up to approximately 3 years. Despite the initial efficacy of EGFR-TKIs, acquired resistance leading to disease progression occurs in almost all patients. The T790M mutation, a major mechanism of acquired resistance to the first line EGFR-TKIs, now could be overcome because of the development of new generation EGFR-TKI targeting the T790M mutation, osimertinib, which has been approved recently. However, the next additive mutation C797S has been reported as a resistant mechanism to osimertinib that can’t be controlled by clinically relevant EGFR-TKIs. The therapeutic strategy against C797S+T790M would become a big concern considering the near future situation that the number of patient with C797S+T790M resistance mutation might rapidly increase, as more and more patients are administered osimertinib and occurrence rate of C797S within patients who receive osimertinib is now estimated as about over 20% (Figure 1).
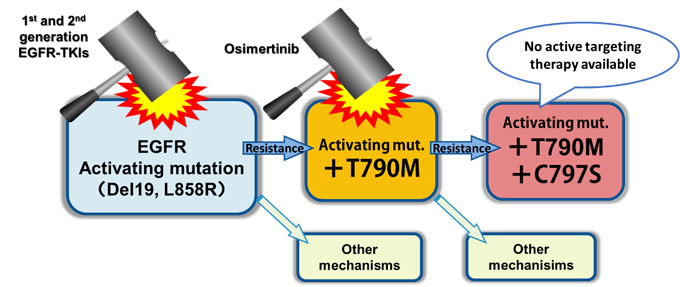
- Figure 1: The treatment strategy of EGFR-TKI positive lung cancer; The 1st and 2nd generation EGFR-TKIs are recommended as the 1st line. These drugs fail to control the disease when resistance such as T790M mutation (accounting for 50% of resistant cases) is acquired, while osimertinib is effective to the T790M mutation. However, an additive C797S mutation (T790M + C797S) leads to the resistance against osimertinib. Now, no active targeting therapy to C797S is available.
We discovered that brigatinib, an ALK tyrosine kinase inhibitor now in late phase clinical development, shows inhibitory activity to the C797S/T790M/activating-mutated (triple-mutated) EGFR. The docking simulation using “K computer” demonstrated that brigatinib fits into the ATP-binding pocket of triple mutant EGFR protein. The structure–activity relationship analysis revealed the key component in brigatinib to inhibit the triple-mutant EGFR that will contribute to the future development of the novel targeting drug. Additionally, we performed in vivo experiment using triple-mutant EGFR positive xenograft bearing mouse and showed that the efficacy of brigatinib to triple-mutation was potentiated in combination with anti-EGFR antibody such as cetuximab or panitumumab that has been utilized clinically (Figure 2). We expect the possibility of evaluating the combination therapy in clinical trial for further study.
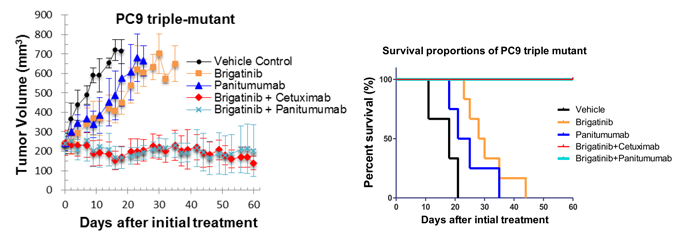
- Figure 2: The xenograft model of triple-mutation (C797S/T790M/activating mutation) cells bearing mice; The mice are treated with indicated treatments. The combination of brigatinib and cetuximab or panitumumab showed significantly durable tumor control that resulted in the prolongation of survival period of mice when compared with vehicle control or monotherapy of brigatinib or panitumumab.





