- HOME
- Outstanding Progress
Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer
Bo Gong1,2, Kazuma Kiyotani3, Seiji Sakata4, Seiji Nagano5,6, Shun Kumehara5,6, Satoko Baba4, Benjamin Besse7,8, Noriko Yanagitani9, Luc Friboulet7, Makoto Nishio9, Kengo Takeuchi4,10, Hiroshi Kawamoto5, Naoya Fujita1,2, Ryohei Katayama1*
JOURNAL OF EXPERIMENTAL MEDICINE, 2019
1 Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
2 Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa-shi, Chiba, 277-8561, JAPAN.
3 Immunopharmacogenomics Group, Cancer Precision Medicine Center, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
4 Pathology Project for Molecular Targets, Cancer Institute, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
5 Laboratory of Immunology, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawaramachi, Syogoinn, Sakyo-ku, Kyoto, JAPAN.
6 Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 53 Kawaramachi, Syogoinn, Sakyo-ku, Kyoto, JAPAN.
7 INSERM U981, Gustave Roussy Cancer Campus, Université Paris Saclay, Villejuif, France.
8 Department of Cancer Medicine, Gustave RoussyCancer Campus, Villejuif, France
9 The Cancer Institute Hospital, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
10 Division of Pathology, Cancer Institute, Japanese Foundation for Cancer Research, 3-8-31, Ariake, Koto-ku, Tokyo 135-8550, JAPAN.
In general, immune system in our body will protect us from infection or carcinogenesis through recognizing and eliminating foreign pathogens, such as bacteria and viruses, or pre-cancerous and cancerous cells. Immune checkpoint molecules including programmed cell death 1 (PD-1) and its ligand (PD-L1) have been revealed to promote cancer survival by suppressing the function of immune cells, especially cytotoxic T cells (CTLs). Recently, immune checkpoint blockade antibody therapies against PD-1 or PD-L1 have been widely used and shown remarkable tumor shrinkage or durable disease control in various cancer. However, therapeutic resistance after initial response is increasingly observed, and the mechanisms underlying anti-PD-L1 blockade antibody (aPD-L1) treatment have not been clarified yet.
In current study, we identified two unique secreted PD-L1 splicing variants from aPD-L1-resistant NSCLC patients. These secreted PD-L1 variants worked as “decoys” of aPD-L1 antibody in HLA-matched co-culture system of iPSC-derived CD8 T cells and cancer cells. Importantly, expression of secreted PD-L1 variant in cancer cells induced an accumulation of soluble PD-L1 in plasma, and mediated therapeutic resistance to PD-L1 blockade in the MC38 syngeneic mice model (Figure 1). Moreover, PD-1 blockade antibody but not PD-L1 antibody retained its inhibitory activity in the presence of secreted PD-L1 variants. These results suggested that the anti-PD-1 antibody might overcome the secreted PD-L1 variant–induced resistance in vitro and in vivo.
Our finding revealed novel mechanisms for the resistance to PD-L1 blockade antibodies and anti-PD-1 treatment could be a therapeutic option to overcome the resistance mediated by secreted PD-L1 variants (Figure 2).
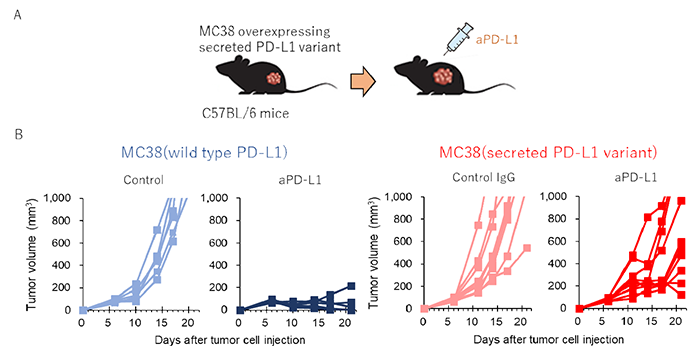
Figure 1: Secreted PD-L1 splicing variants mediate the resistance to PD-L1 blockade in MC38 syngeneic mice mode
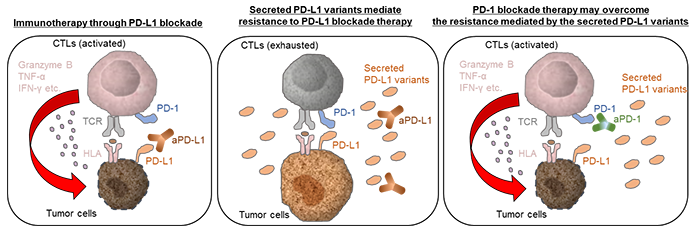
Figure 2: The proposed model for the sPD-L1 splicing variants associated with resistance to aPD-L1 antibody treatment.





