
Advanced Medical Development Center
About | Divisions | Doctors and Staff
Greetings from the enter chief

MD, PhD, FRCS
The Cancer Institute Hospital is equipped with the most advanced treatments for the fight against cancer.Surgery, which has been the main specialty in our history, is undoubtedly a powerful intervention. However, surgery is by no means the only treatment option we rely on. We can improve our chances of winning the battle against cancer if we skillfully use novel chemotherapeutic and immunotherapy agents in conjunction with radiation therapies.
At the Cancer Institute Hospital, the "Clinical Research and Development Center" has carried out many clinical trials to ensure that patients can access newly developed agents under their national health coverage. Furthermore, we have established the "Advanced Medical Development Center", which specializes in the early stages of drug development to promote early phase clinical trials, including offering drugs under pre-clinical development to patients with national health coverage, thus providing patients with cutting-edge cancer care.
Center Chief, Takeshi Sano
Overview and purpose of the center
As the population ages, the number of cancer patients in Japan increases, so the need for new treatments is growing. With the recent development of various new cancer drugs, early clinical development, which is the starting point for development, is becoming more and more competitive internationally. For this reason, the Advanced Medical Development Center was established at the Cancer Institute Hospital to serve as a clinical development base for the next generation of cancer therapies, with the aim of bringing new cancer drugs from Japan to patients around the world as quickly as possible.
The emergence of cancer drugs with complex mechanisms of action (the mechanisms by which drugs exert therapeutic effects), such as immunotherapy, and the individualization of target patients using genetic diagnosis, have led to the need for a wide range of expertise and experience in the development of new cancer drugs. For this reason, the center has established three divisions: the “Division of Early Clinical Development for Cancer”, the “Division of Cancer Immunotherapy Development”, and the “Division of Cancer Genomic Medicine Development”. In addition to bringing together knowledge in these specialized fields, we will promote innovative early clinical development by proposing clinical trials suitable for individual patients. This work will involve collaboration between the departments and divisions of the Cancer Institute Hospital.
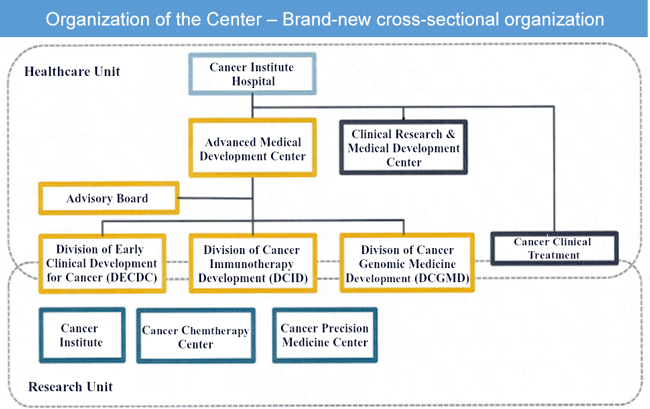
- Division of Early Clinical Development for Cancer (DECDC)
We promote early clinical development of low-molecular drugs and antibody drugs for various cancers and phase I trials, including first-in-human trials. - Division of Cancer Immunotherapy Development (DCID)
We carry out early clinical development of cancer immunotherapeutic drugs, such as immune-checkpoint inhibitors, gene-engineering cell therapies, and combination therapy with various drugs (combined cancer immunotherapy). - Division of Cancer Genomic Medicine Development (DCGMD)
We conduct clinical trials to implement in vitro diagnostics using advanced genomic analysis developed at the Cancer Precision Medicine Center (CPM Center) to promote advanced medicine.
With our staff’s expertise in cancer treatment, we also provide second opinions to cancer
patients treated at medical institutions other than the Cancer Institute Hospital.


