
���B�Z���^�[
��p�E������
���B�O��
��p�����i2024�N�j
| �����p���i���@+�O���j | 1,202 |
|---|---|
| �@���[���� | 380 |
| �@���[�؏� | 821 |
| �@������������ | 213 |
| ���[����Ȃ� | 1 |
| ���̑� | 182 |
| �@�\�h�I�؏� | 43 |
| �@�ǐ���p | 27 |
| �@�Ĕ���p | 43 |
| �@�lj���p | 46 |
| �@��L�ȊO | 23 |
| �v | 1,384 |
��p������
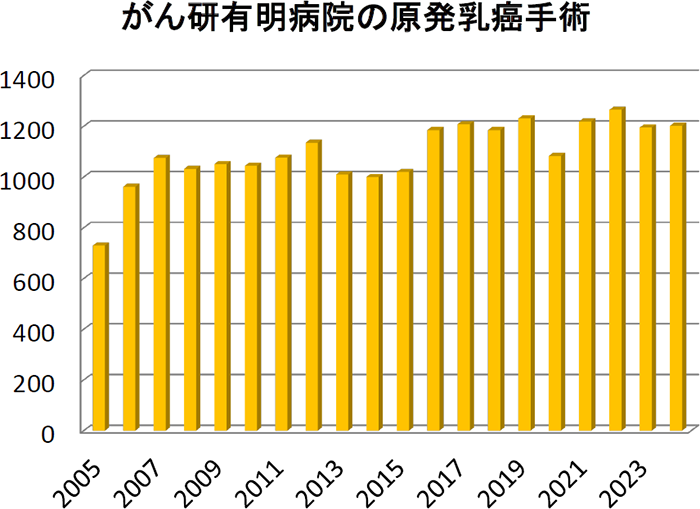
���B����
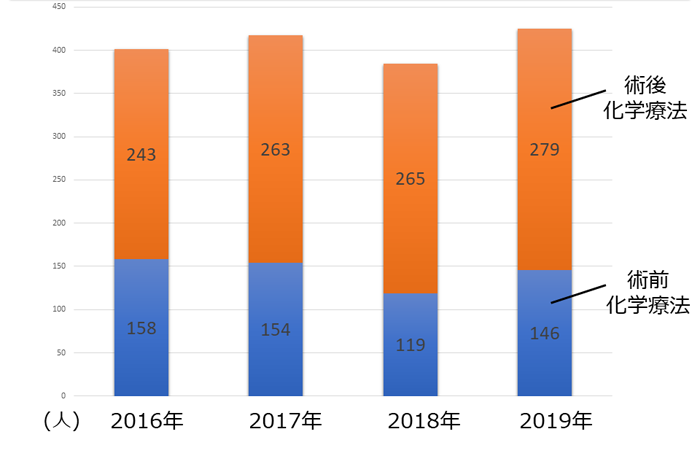
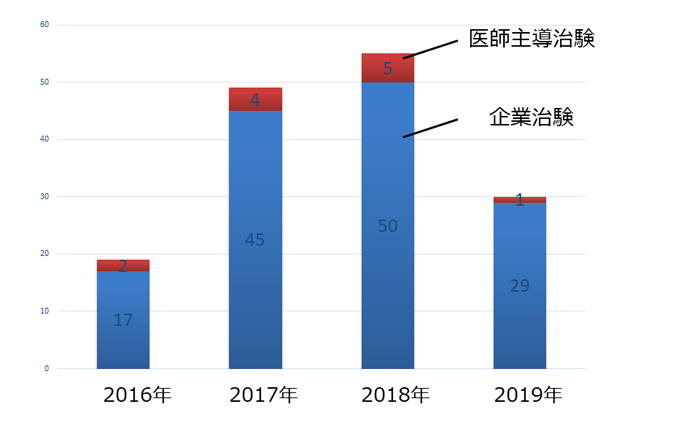
�N�ԐV�K���Ґ��i�p�O�E�p��j
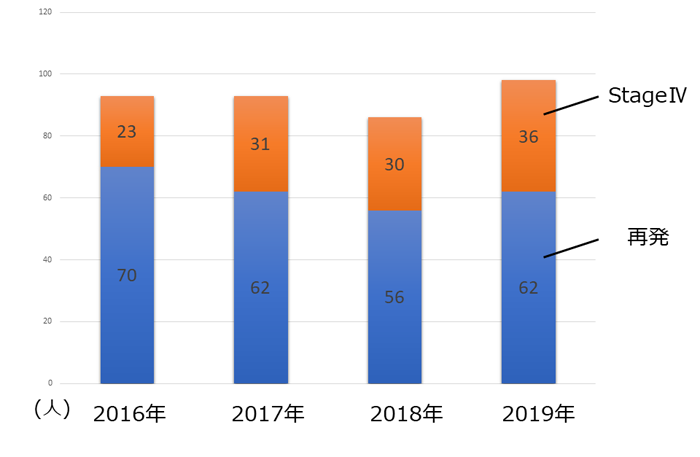
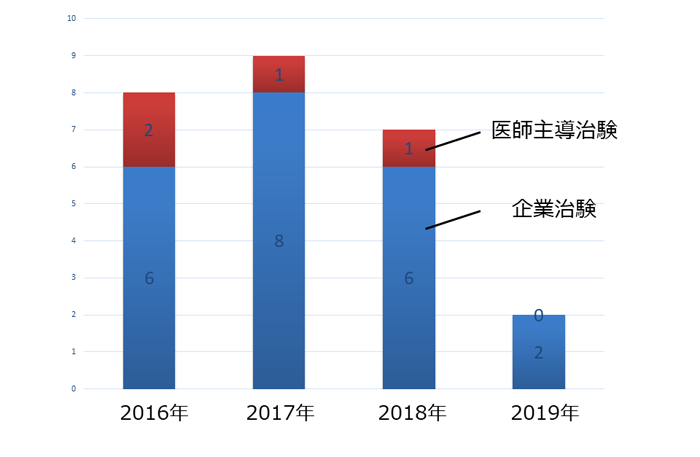
�N�ԐV�K���Ґ��i�Ĕ��EStageIV�j
�N�ԍĐf���Ґ��i���ב����j
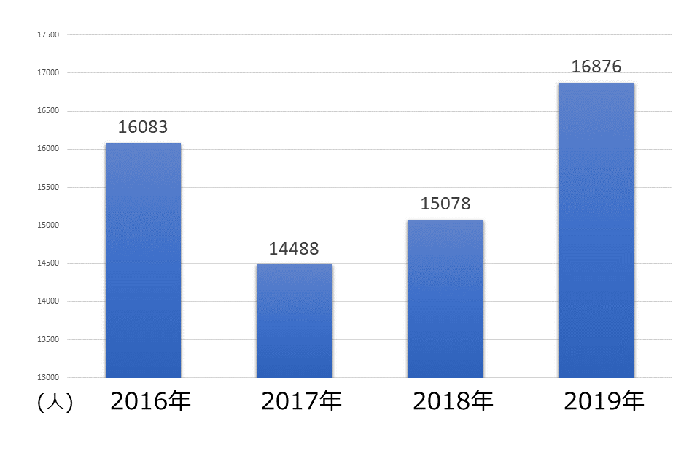
�N�ԉғ���������
�V�K�����������
���B�Z���^�[��������
2024�N
Nogi, H., Ogiya, A., Ishitobi, M., Yamauchi, C., Mori, H., Shimo, A., Narui, K., Nagura, N., Seki, H., Sasada, S., Sakurai, T., Shien, T.
Impact of neoadjuvant chemotherapy on the safety and long-term outcomes of patients undergoing immediate breast reconstruction after mastectomy. Breast Cancer, 31:507-518, 2024
Abe, T., Kataoka, A., Uehiro, N., Yoshida, N., Nishimura, M., Ozaki, Y., Kogawa, T., Takano, T., Ohno, S., Ueno, T.
Desire for pregnancy and fertility preservation in young patients with breast cancer. Breast Cancer, 31: 1137-1143, 2024
Abe, A., Nomura, H., Fusegi, A., Yunokawa, M., Ueki, A., Habano, E., Arakawa, H., Kaneko, K., Minoura, Y., Inari, H., Ueno, T., Kanao, H.
Risk-reducing decisions regarding germline BRCA pathogenic variant: Focusing on the timing of genetic testing and RRSO. J Med Genet, 61: 392-398, 2024
Aoyama, Y., Ozaki, Y., Kizawa, R., Masuda, J., Kawai, S., Kurata, M., Maeda, T., Yoshida, K., Yamashita, N., Nishimura, M., Hosonaga, M., Fukada, I., Hara, F., Kobayashi, T., Takano, T., Ueno, T.
Efficacy and feasibility of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative and estrogen receptor low, HER2-negative breast cancer: a Japanese single-institution real-world study. Breast Cancer: in press, 2024
Azim, H. A., Jr., Niman, S. M., Partridge, A. H., Demeestere, I., Ruggeri, M., Colleoni, M., Saura, C., Shimizu, C., Saetersdal, A. B., Kroep, J. R., Mailliez, A., Warner, E., Borges, V. F., Amant, F., Gombos, A., Kataoka, A., Rousset-Jablonski, C., Borstnar, S., Takei, J., Lee, J. E., Walshe, J. M., Ruíz-Borrego, M., Moore, H. C. F., Saunders, C., Bjelic-Radisic, V., Susnjar, S., Cardoso, F., Klar, N. J., Spanic, T., Ruddy, K., Piccart, M., Korde, L. A., Goldhirsch, A., Gelber, R. D., Pagani, O., Peccatori, F. A.
Fertility Preservation and Assisted Reproduction in Patients With Breast Cancer Interrupting Adjuvant Endocrine Therapy to Attempt Pregnancy. J. Clin. Oncol., 42: 2822-2832, 2024
Baba, T., Kusumoto, M., Kato, T., Kurihara, Y., Sasaki, S., Oikado, K., Saito, Y., Endo, M., Fujiwara, Y., Kenmotsu, H., Sata, M., Takano, T., Kato, K., Hirata, K., Katagiri, T., Saito, H., Kuwano, K.
Correction to: Clinical and imaging features of interstitial lung disease in cancer patients treated with trastuzumab deruxtecan. Int J Clin Oncol, 29: 78-79, 2024
Bhattacharya, A., Wang, K., Penailillo, J., Chan, C. N., Fushimi, A., Yamashita, N., Daimon, T., Haratake, N., Ozawa, H., Nakashoji, A., Shigeta, K., Morimoto, Y., Miyo, M., Kufe, D. W.
MUC1-C regulates NEAT1 lncRNA expression and paraspeckle formation in cancer progression. Oncogene, 43: 2199-2214, 2024
Cardoso, F., Paluch-Shimon, S., Schumacher-Wulf, E., Matos, L., Gelmon, K., Aapro, M. S., Bajpai, J., Barrios, C. H., Bergh, J., Bergsten-Nordström, E., Biganzoli, L., Cardoso, M. J., Carey, L. A., Mac Gregor, M. C., Chidebe, R., Cortés, J., Curigliano, G., Dent, R. A., El Saghir, N. S., Eniu, A., Fallowfield, L., Francis, P. A., Franco Millan, S. X., Gilchrist, J., Gligorov, J., Gradishar, W. J., Haidinger, R., Harbeck, N., Hu, X., Kaur, R., Kiely, B., Kim, S. B., Koppikar, S., Kuper-Hommel, M. J. J., Lecouvet, F. E., Mason, G., Mertz, S. A., Mueller, V., Myerson, C., Neciosup, S., Offersen, B. V., Ohno, S., Pagani, O., Partridge, A. H., Penault-Llorca, F., Prat, A., Rugo, H. S., Senkus, E., Sledge, G. W., Swain, S. M., Thomssen, C., Vorobiof, D. A., Vuylsteke, P., Wiseman, T., Xu, B., Costa, A., Norton, L., Winer, E. P.
6th and 7th International consensus guidelines for the management of advanced breast cancer (ABC guidelines 6 and 7). Breast, 76: 103756, 2024
Daimon, T., Bhattacharya, A., Wang, K., Haratake, N., Nakashoji, A., Ozawa, H., Morimoto, Y., Yamashita, N., Kosaka, T., Oya, M., Kufe, D. W.
MUC1-C is a target of salinomycin in inducing ferroptosis of cancer stem cells. Cell Death Discov., 10: 9, 2024
Fehm, T., Cottone, F., Dunton, K., André, F., Krop, I., Park, Y. H., De Laurentiis, M., Miyoshi, Y., Armstrong, A., Borrego, M. R., Yerushalmi, R., Duhoux, F. P., Takano, T., Lu, W., Egorov, A., Kim, S. B.
Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): patient-reported outcomes from a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol., 25: 614-625, 2024
Ganz, P. A., Bandos, H., Španić, T., Friedman, S., Müller, V., Kuemmel, S., Delaloge, S., Brain, E., Toi, M., Yamauchi, H., de Dueñas, E. M., Armstrong, A., Im, S. A., Song, C. G., Zheng, H., Sarosiek, T., Sharma, P., Geng, C., Fu, P., Rhiem, K., Frauchiger-Heuer, H., Wimberger, P., t'Kint de Roodenbeke, D., Liao, N., Goodwin, A., Chakiba-Brugère, C., Friedlander, M., Lee, K. S., Giacchetti, S., Takano, T., Henao-Carrasco, F., Virani, S., Valdes-Albini, F., Domchek, S. M., Bane, C., McCarron, E. C., Mita, M., Rossi, G., Rastogi, P., Fielding, A., Gelber, R. D., Scheepers, E. D., Cameron, D., Garber, J., Geyer, C. E., Tutt, A. N. J.
Patient-reported outcomes in OlympiA: A phase III, randomized, placebo-controlled trial of adjuvant olaparib in gBRCA1/2 mutations and high-risk human epidermal growth factor receptor 2-negative early breast cancer. J. Clin. Oncol., 42: 1288-1300, 2024
Hara, F., Nagasaki, R., Minami, R., Izutani, T., Yoshida, T., Arai, A., Nihei, A., Sakurai, N., Ohno, S.
Survey on adverse events associated with drug therapy for breast cancer patients. BMC women's health, 24: 545, 2024
Hattori, M., Honma, N., Nagai, S., Narui, K., Shigechi, T., Ozaki, Y., Yoshida, M., Sakatani, T., Sasaki, E., Tanabe, Y., Tsurutani, J., Takano, T., Saji, S., Masuda, S., Horii, R., Tsuda, H., Yamaguchi, R., Toyama, T., Yamauchi, C., Toi, M., Yamamoto, Y.
Trastuzumab deruxtecan for human epidermal growth factor receptor 2-low advanced or metastatic breast cancer: recommendations from the Japanese Breast Cancer Society Clinical Practice Guidelines. Breast Cancer, 31: 335-339, 2024
Hayashi, N., Ono, M., Fukada, I., Yamazaki, M., Sato, N., Hosonaga, M., Wang, X., Kaneko, K., Arakawa, H., Habano, E., Kuga, A., Kataoka, A., Ueki, A., Kiyotani, K., Tonooka, A., Takeuchi, K., Kogawa, T., Kitano, S., Takano, T., Watanabe, M., Mori, S., Takahashi, S.
Addressing the knowledge gap in the genomic landscape and tailored therapeutic approaches to adolescent and young adult cancers. ESMO Open, 9: 103659, 2024
Hayashi, N., Mori, S., Ohmoto, A., Fukada, I., Yamazaki, M., Hosonaga, M., Wang, X., Ueki, A., Kiyotani, K., Tonooka, A., Takeuchi, K., Takahashi, S.
Availability of genome-matched therapy based on clinical practice. Int J Clin Oncol, 29: 964-971, 2024
Hirose, T., Ito, M., Tsuchihashi, K., Ozaki, Y., Nishio, H., Ichihara, E., Miura, Y., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Endo, M.
Primary prophylaxis with G-CSF for patients with non-round cell soft tissue sarcomas: a systematic review for the Clinical Practice Guidelines for the Use of G-CSF 2022 of the Japan Society of Clinical Oncology. Int J Clin Oncol, 29: 1067-1073, 2024
Hirose, T., Ito, M., Tsuchihashi, K., Ozaki, Y., Nishio, H., Ichihara, E., Miura, Y., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Endo, M.
Effectiveness and safety of primary prophylaxis with G-CSF for patients with Ewing sarcomas: a systematic review for the Clinical Practice Guidelines for the Use of G-CSF 2022 of the Japan Society of Clinical Oncology. Int J Clin Oncol, 29: 1081-1087, 2024
Hosonaga, M., Habano, E., Arakawa, H., Kaneko, K., Nakajima, T., Hayashi, N., Fukada, I., Nakamura, A., Haruyama, Y., Maeda, T., Inari, H., Kobayashi, T., Nakashima, E., Ueno, T., Takano, T., Takahashi, S., Ohno, S., Ueki, A.
Case series of Li-Fraumeni syndrome: carcinogenic mechanisms in breast cancer with TP53 pathogenic variant carriers. Breast Cancer, 31: 988-996, 2024
Ichihara, E., Ochi, N., Makimoto, G., Kudo, K., Harada, D., Ozaki, Y., Nishio, H., Tsuchihashi, K., Miura, Y., Endo, M., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Kubo, T.
Effectiveness and safety of primary prophylaxis with G-CSF for lung cancer: a systematic review and meta-analysis to develop clinical practice guidelines for the use of G-CSF 2022. Int J Clin Oncol, 29: 355-362, 2024
Iesato, A., Fushimi, A., Tahara, R., Terada, M., Iwase, M., Kawamura, C., Yamashita, N.
A novel system to provide information via online YouTube videos and an evaluation of current online information about hereditary breast cancer. Breast Cancer, 31: 63-74, 2024
Im, S. A., Cortes, J., Cescon, D. W., Yusof, M. M., Iwata, H., Masuda, N., Takano, T., Huang, C. S., Chung, C. F., Tsugawa, K., Park, Y. H., Matsumoto, K., Inoue, K., Kwong, A., Loi, S., Fu, W., Pan, W., Karantza, V., Rugo, H. S., Schmid, P.
Results from the randomized KEYNOTE-355 study of pembrolizumab plus chemotherapy for Asian patients with advanced TNBC. npj Breast Cancer, 10: 79, 2024
Ito, M., Okumura, Y., Nio, K., Baba, E., Ozaki, Y., Nishio, H., Ichihara, E., Miura, Y., Endo, M., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Tsuchihashi, K.
Effectiveness of G-CSF in chemotherapy for digestive system tumors: a systematic review of the Clinical Practice Guidelines for the Use of G-CSF 2022 delineated by the Japan Society of Clinical Oncology. Int J Clin Oncol, 29: 689-699, 2024
Iwamoto, T., Niikura, N., Watanabe, K., Takeshita, T., Kikawa, Y., Kobayashi, K., Iwakuma, N., Okamura, T., Kobayashi, T., Katagiri, Y., Kitada, M., Tomioka, N., Miyoshi, Y., Shigematsu, H., Miyashita, M., Ishiguro, H., Masuda, N., Saji, S.
Prognostic value of the 21-Gene Breast Recurrence Score® assay for hormone receptor-positive/human epidermal growth factor 2-negative advanced breast cancer: subanalysis from Japan Breast Cancer Research Group-M07 (FUTURE trial). Breast Cancer Res. Treat., 208: 253-262, 2024
Kato, S., Mori, H., Saiga, M., Watanabe, S., Sasada, S., Sasaki, A., Ogiya, A., Yamamoto, M., Narui, K., Takano, J., Seki, H., Nagura, N., Ishitobi, M., Shien, T.
Nipple-areolar complex malposition in breast reconstruction after nipple-sparing mastectomy: a multi-institutional retrospective observational study in Japan. Breast Cancer, 31: 649-658, 2024
Kim, J., Kim, J., Seo, K. H., Lee, K. H., Park, Y. H., Lin, C. H., Lu, Y. S., Ueno, T., Yap, Y. S., Wong, F. Y., Tan, V. K. M., Lim, G. H., Tan, S. M., Yeo, W., Liu, Q., Leung, R., Naito, Y., Li, H., Lee, H. B., Han, W., Im, S. A.
Survival outcomes of young-age female patients with early breast cancer: an international multicenter cohort study. ESMO Open, 9: 103732, 2024
Kimura, S., Shigeta, K., Tamura, S., Uchino, K., Kimura, T., Ozaki, Y., Nishio, H., Tsuchihashi, K., Ichihara, E., Endo, M., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Miura, Y.
Effectiveness and safety of primary prophylaxis of G-CSF during chemotherapy for prostate cancer, Japanese clinical guideline for appropriate use of G-CSF: clinical practice guidelines for the use of G-CSF 2022. Int J Clin Oncol, 29: 559-563, 2024
Kobayashi, T., Nishimura, M., Hosonaga, M., Kizawa, R., Kawai, S., Aoyama, Y., Ozaki, Y., Fukada, I., Hara, F., Takano, T., Ueno, T.
Absolute lymphocyte count predicts efficacy of palbociclib in patients with metastatic luminal breast cancer. BMC Cancer, 24: 1156, 2024
Kojima, R., Ishitobi, M., Nagura, N., Shimo, A., Seki, H., Ogiya, A., Sakurai, T., Seto, Y., Sasada, S., Oshiro, C., Kato, M., Kawate, T., Kondo, N., Shien, T.
Receptor discordance after nipple-sparing mastectomy. Surg Pract Sci, 17: 100239, 2024
Maeda, T., Najima, Y., Kamiyama, Y., Nakao, S., Ozaki, Y., Nishio, H., Tsuchihashi, K., Ichihara, E., Miumra, Y., Endo, M., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Yano, S.
Effectiveness and safety of primary prophylaxis with G-CSF after induction therapy for acute myeloid leukemia: a systematic review and meta-analysis of the clinical practice guidelines for the use of G-CSF 2022 from the Japan society of clinical oncology. Int J Clin Oncol, 29: 535-544, 2024
Najima, Y., Maeda, T., Kamiyama, Y., Nakao, S., Ozaki, Y., Nishio, H., Tsuchihashi, K., Ichihara, E., Miumra, Y., Endo, M., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Yano, S.
Effectiveness and safety of granulocyte colony-stimulating factor priming regimen for acute myeloid leukemia: A systematic review and meta-analysis of the Clinical Practice Guideline for the use of G-CSF 2022 from the Japan Society of Clinical Oncology. Int J Clin Oncol, 29: 899-910, 2024
Nishimura, T., Velaga, R., Masuda, N., Kawaguchi, K., Kawaguchi, S., Takada, M., Maeshima, Y., Tanaka, S., Kikawa, Y., Kadoya, T., Bando, H., Nakamura, R., Yamamoto, Y., Ueno, T., Yasojima, H., Ishiguro, H., Morita, S., Ohno, S., Haga, H., Matsuda, F., Ogawa, S., Toi, M.
Genomic and transcriptomic profiling of pre- and postneoadjuvant chemotherapy triple negative breast cancer tumors. Cancer Sci., 115: 3928-3942, 2024
Nonomiya, Y., Nakayama, I., Kobayashi, K., Amakawa, Y., Shibata, N., Soejima, A., Kawakami, K., Shimizu, H., Takahari, D., Kawai, S., Hara, F., Takano, T., Yamaguchi, K., Yamaguchi, M.
Analysis of Adverse Events Associated with Trastuzumab Deruxtecan in Patients with Gastric and Breast Cancer: A Retrospective Study. Biol. Pharm. Bull., 47: 411-416, 2024
Nozawa, K., Ozaki, Y., Yoshinami, T., Yokoe, T., Nishio, H., Tsuchihashi, K., Ichihara, E., Miura, Y., Endo, M., Yano, S., Maruyama, D., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T.
Effectiveness and safety of primary prophylaxis with G-CSF during chemotherapy for invasive breast cancer: a systematic review and meta-analysis from Clinical Practice Guidelines for the Use of G-CSF 2022. Int J Clin Oncol, 29: 1074–1080, 2024
Ogiya, A., Kimura, K., Ueno, T., Iwase, T., Ohno, S.
Time trend of breast cancer-related lymphedema according to body mass index. Eur. J. Surg. Oncol., 50: 108350, 2024
Otsuji, K., Takahashi, Y., Osako, T., Kobayashi, T., Takano, T., Saeki, S., Yang, L., Baba, S., Kumegawa, K., Suzuki, H., Noda, T., Takeuchi, K., Ohno, S., Ueno, T., Maruyama, R.
Serial single-cell RNA sequencing unveils drug resistance and metastatic traits in stage IV breast cancer. npj Precis Oncol, 8: 222, 2024
Ozaki, Y., Yokoe, T., Yoshinami, T., Nozawa, K., Nishio, H., Tsuchihashi, K., Ichihara, E., Miura, Y., Endo, M., Yano, S., Maruyama, D., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T.
Optimal timing of prophylactic pegylated G-CSF after chemotherapy administration for patients with cancer: a systematic review and meta-analysis from Clinical Practice Guidelines for the use of G-CSF 2022. Int J Clin Oncol, 29: 551-558, 2024
Rediti, M., Venet, D., Joaquin Garcia, A., Maetens, M., Vincent, D., Majjaj, S., El-Abed, S., Di Cosimo, S., Ueno, T., Izquierdo, M., Piccart, M., Pusztai, L., Loi, S., Salgado, R., Viale, G., Rothé, F., Sotiriou, C.
Identification of HER2-positive breast cancer molecular subtypes with potential clinical implications in the ALTTO clinical trial. Nat. Commun., 15: 10402, 2024
Sakai, T., Kutomi, G., Shien, T., Asaga, S., Aruga, T., Ishitobi, M., Kuba, S., Sawaki, M., Terata, K., Tomita, K., Yamauchi, C., Yamamoto, Y., Iwata, H., Saji, S.
The Japanese Breast Cancer Society Clinical Practice Guidelines for surgical treatment of breast cancer, 2022 edition. Breast Cancer, 31: 1-7, 2024
Saldajeno, D. P., Kawaoka, S., Masuda, N., Tanaka, S., Bando, H., Nishimura, T., Kadoya, T., Yamanaka, T., Imoto, S., Velaga, R. M., Tamura, N., Aruga, T., Ikeda, K., Fukui, Y., Maeshima, Y., Takada, M., Suzuki, E., Ueno, T., Ogawa, S., Haga, H., Ohno, S., Morita, S., Kawaguchi, K., Toi, M.
Time-series blood cytokine profiles correlate with treatment responses in triple-negative breast cancer patients. Br J Cancer, 130: 1023-1035, 2024
Sasada, S., Nagura, N., Shimo, A., Ogiya, A., Saiga, M., Seki, H., Mori, H., Kondo, N., Ishitobi, M., Narui, K., Nogi, H., Yamauchi, C., Sakurai, T., Shien, T.
Impact of radiation therapy for breast cancer with involved surgical margin after immediate breast reconstruction: A multi-institutional observational study. Eur. J. Surg. Oncol., 50: 108360, 2024
Seki, H., Ogiya, A., Nagura, N., Shimo, A., Narui, K., Sasada, S., Ishitobi, M., Nogi, H., Kondo, N., Sakurai, T., Yamauchi, C., Mori, H., Saiga, M., Niikura, N., Shien, T.
Prognosis of isolated locoregional recurrence after early breast cancer with immediate breast reconstruction surgery: a retrospective multi-institutional study. Breast Cancer, 31: 935-944, 2024
Shien, T., Tsuda, H., Sasaki, K., Mizusawa, J., Akiyama, F., Kurosumi, M., Sawaki, M., Tamura, N., Tanaka, K., Kogawa, T., Takahashi, M., Hayashi, N., Mukai, H., Masuda, N., Hara, F., Iwata, H.
Comparison of proportions and prognostic impact of pathological complete response between evaluations of representative specimen and total specimen in primary breast cancer after neoadjuvant chemoradiotherapy: an ancillary study of JCOG0306. Breast Cancer Res. Treat., 208: 145-154, 2024
Suzuki, K., Yokokawa, T., Kawaguchi, T., Takada, S., Tamaki, S., Kawasaki, Y., Yamaguchi, T., Koizumi, K., Matsumoto, T., Sakata, Y., Arakawa, Y., Ayuhara, H., Hosonaga, M., Yamaguchi, M., Tsuji, D.
A multicenter, phase II trial of triplet antiemetic therapy with palonosetron, aprepitant, and olanzapine for highly emetogenic chemotherapy in breast cancer (PATROL-II) Sci. Rep., 14: 28271, 2024
Taira, N., Kikawa, Y., Iwamoto, T., Miyoshi, Y., Hara, K., Yoshitomi, S., Hikino, H., Takahashi, H., Takabatake, D., Kubo, S., Ikeda, M., Doihara, H., Shien, T., Okuyama, H., Tanabe, Y., Hara, F., Yamanouchi, K., Hagiwara, Y., Sawaki, M.
Pilot trial of an electronic patient-reported outcome monitoring system in patients with metastatic breast cancer undergoing chemotherapy. Breast Cancer, 31: 283-294, 2024
Takahashi, S., Sato, N., Kaneko, K., Masuda, N., Kawai, M., Hirakawa, H., Nomizu, T., Iwata, H., Ueda, A., Ishikawa, T., Bando, H., Inoue, Y., Ueno, T., Ohno, S., Kubo, M., Yamauchi, H., Okamoto, M., Tokunaga, E., Kamigaki, S., Aogi, K., Komatsu, H., Kitada, M., Uemoto, Y., Toyama, T., Yamamoto, Y., Yamashita, T., Yanagawa, T., Yamashita, H., Matsumoto, Y., Toi, M., Miyashita, M., Ishida, T., Fujishima, F., Sato, S., Yamaguchi, T., Takahashi, F., Ishioka, C.
TP53 signature predicts pathological complete response after neoadjuvant chemotherapy for breast cancer: Observational and confirmational study using prospective study cohorts. Transl. Oncol., 48: 102060, 2024
Takano, T., Masuda, N., Ito, M., Inoue, K., Tanabe, Y., Kawaguchi, K., Yasojima, H., Bando, H., Nakamura, R., Yamanaka, T., Ishida, K., Aruga, T., Yanagita, Y., Tokunaga, E., Aogi, K., Ohno, S., Kasai, H., Kataoka, T. R., Morita, S., Toi, M.
Long-term outcomes of neoadjuvant trastuzumab emtansine + pertuzumab (T-DM1 + P) and docetaxel + carboplatin + trastuzumab + pertuzumab (TCbHP) for HER2-positive primary breast cancer: results of the randomized phase 2 JBCRG20 study (Neo-peaks). Breast Cancer Res. Treat., 207: 33-48, 2024
Tan, R. Y. C., Ong, W. S., Lee, K. H., Park, S., Iqbal, J., Park, Y. H., Lee, J. E., Yu, J. H., Lin, C. H., Lu, Y. S., Ono, M., Ueno, T., Naito, Y., Onishi, T., Lim, G. H., Tan, S. M., Lee, H. B., Koh, J., Han, W., Im, S. A., Tan, V. K. M., Phyu, N., Wong, F. Y., Tan, P. H., Yap, Y. S.
Outcomes in Nonmetastatic Hormone Receptor-Positive HER2-Negative Pure Mucinous Breast Cancer: A Multicenter Cohort Study. J. Natl. Compr. Canc. Netw, 22: e237121, 2024
Tolaney, S. M., DeMichele, A., Takano, T., Rugo, H. S., Perou, C., Lynce, F., Parsons, H. A., Santa-Maria, C. A., Rocque, G. B., Yao, W., Sun, S. W., Mocci, S., Partridge, A. H., Carey, L. A.
OptimICE-RD: sacituzumab govitecan + pembrolizumab vs pembrolizumab (± capecitabine) for residual triple-negative breast cancer. Future Oncol., 20: 2343-2355, 2024
Tomomatsu, T., Shimizu, H., Yokokawa, T., Fukada, I., Kawakami, K., Kobayashi, K., Aoyama, T., Suzuki, W., Sugisaki, T., Hashimoto, K., Asano, M., Mori, Y., Hara, F., Takano, T., Ohno, S., Yamaguchi, M.
Cost of Pegfilgrastim for the Prophylaxis of Chemotherapy-induced Febrile Neutropenia in Patients with Breast Cancer Receiving Perioperative Chemotherapy in Daily Practice in Japan. YAKUGAKU ZASSHI, 144: 897-904, 2024
Tsuchida, Y., Niikura, N., Chishima, T., Mizuno, M., Kawate, T., Fuchikami, H., Miyoshi, Y., Sakai, T., Kotani, H., Kondo, N., Hayashi, N.
Correlation between postoperative treatment selection and prognosis determined using the Oncotype DX® test data: a retrospective multicenter study in Japan. Breast Cancer, 31: 401-408, 2024
Tsuchihashi, K., Ito, M., Okumura, Y., Nio, K., Ozaki, Y., Nishio, H., Ichihara, E., Miura, Y., Endo, M., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Baba, E.
Therapeutic use of granulocyte colony-stimulating factor (G-CSF) in patients with febrile neutropenia: a comprehensive systematic review for clinical practice guidelines for the use of G-CSF 2022 from the Japan Society of Clinical Oncology. Int J Clin Oncol, 29: 700-705, 2024
Uchino, K., Tamura, S., Kimura, S., Shigeta, K., Kimura, T., Ozaki, Y., Nishio, H., Tsuchihashi, K., Ichihara, E., Endo, M., Yano, S., Maruyama, D., Yoshinami, T., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T., Miura, Y.
Effectiveness and safety of primary prophylaxis of granulocyte colony-stimulating factor during dose-dense chemotherapy for urothelial cancer: Clinical Practice Guidelines for the use of G-CSF 2022. Int J Clin Oncol, 29: 545-550, 2024
Yamamoto, Y., Yamauchi, C., Toyama, T., Nagai, S., Sakai, T., Kutomi, G., Yoshimura, M., Kawai, M., Ohtani, S., Kubota, K., Nakashima, K., Honma, N., Yoshida, M., Tokunaga, E., Taira, N., Iwata, H., Saji, S.
The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer, 2022 Edition: changes from the 2018 edition and general statements on breast cancer treatment. Breast Cancer, 31: 340-346, 2024
Yamamoto, Y., Yamauchi, C., Toyama, T., Nagai, S., Sakai, T., Kutomi, G., Yoshimura, M., Kawai, M., Ohtani, S., Kubota, K., Nakashima, K., Honma, N., Yoshida, M., Tokunaga, E., Taira, N., Iwata, H., Saji, S.
Correction: The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer, 2022 Edition: changes from the 2018 edition and general statements on breast cancer treatment. Breast Cancer, 31: 736-737, 2024
Yang, L., Kumegawa, K., Saeki, S., Nakadai, T., Maruyama, R.
Identification of lineage-specific epigenetic regulators FOXA1 and GRHL2 through chromatin accessibility profiling in breast cancer cell lines. Cancer Gene Ther., 31: 736-745, 2024
Yoshida, R., Kaneyasu, T., Ueki, A., Yamauchi, H., Ohsumi, S., Ohno, S., Aoki, D., Baba, S., Kawano, J., Matsumoto, N., Nagasaki, M., Ueno, T., Inari, H., Kobayashi, Y., Takei, J., Gotoh, O., Nishi, M., Okamura, M., Kaneko, K., Okawa, M., Suzuki, M., Amino, S., Inuzuka, M., Noda, T., Mori, S., Nakamura, S.
High-risk pathogenic germline variants in blood relatives of BRCA1/2 negative probands. Breast Cancer, 31: 1028-1036, 2024
Yoshinami, T., Nozawa, K., Yokoe, T., Ozaki, Y., Nishio, H., Tsuchihashi, K., Ichihara, E., Miura, Y., Endo, M., Yano, S., Maruyama, D., Susumu, N., Takekuma, M., Motohashi, T., Ito, M., Baba, E., Ochi, N., Kubo, T., Uchino, K., Kimura, T., Kamiyama, Y., Nakao, S., Tamura, S., Nishimoto, H., Kato, Y., Sato, A., Takano, T.
Comparison between a single dose of PEG G-CSF and multiple doses of non-PEG G-CSF: a systematic review and meta-analysis from Clinical Practice Guidelines for the use of G-CSF 2022. Int J Clin Oncol, 29: 681-688, 2024
�O�����, ���ЕF, �O�c�N��, �����m�q, �t�R�D���b, ���L��, �Љ�����, ���, ���J�N�q, �{��R��, ���M�V, ���q�i��, �A�ؗL��, �e�r�^��, �唗�q, ���엘��, ���^�i
Li-Fraumeni�nj�Q�̃T�[�x�C�����X���ɐf�f����������1��. �����̗Տ�, 39: 291-297, 2024
���M�V
Women's Imaging 2024 Breast Imaging Vol.19�@������f�Â̐V�����f�f�Ǝ��Â𗝉�����F������f�Âɂ�����V���Ȑf�f�Z�p�𗝉�����@���L�b�h�o�C�I�v�V�[�̊T�v. INNERVISION, 39: 48-50, 2024
���M�V
�����̂��ׂ�2024�F�\�h�E�f�f�̐i���@�����̃o�C�I���W�[�@�Q�m����Â̎��_����. ��w�̂����, 290: 355-358, 2024
������, ���J�N�q, �唗�q, �ێR��, �|������, ���M�V, ���^�i
���B�����㌴���Ɖu�s�S�֘A�����p���B�������ƑΑ��������ɔF�߂�1��. �����̗Տ�, 39: 85-92, 2024
���L��, ��؈�m, �������, ���їm�q, ���R����, ������, �����F��, ���X�ؐ���, ��������, �g�c�a��, �O�c�N��, �����m�q, ���J�N�q, �{��R��, ������, ���엘��, ���M�V, ���^�i, �������F
AI�z�X�s�^�����Ƃɂ�����C���t�H�[���h�R���Z���g���x���\�����[�V�����J������. �����̗Տ�, 39: 255-266, 2024
���L��
�ŐV�̐f�f�Ǝ��ÁF���[�̎�p�@��G�m�����̎�p�ɂ�����a�ϕ��ʓ���̍H�v. ���{�Տ�, 82: 221-224, 2024
���ЕF
�������Âɂ������p�̏ȗ��ɂ��čl����F���|�����p�ߊs���ȗ��ɂ�����Targeted Axillary Dissection�̌���. ���{�O�Ȋw��G��, 125: 435-442, 2024
���c�~
������f�Â̖����F������Ɖu�Ö@�̖���. ��ᇓ���, 34: 164-172, 2024
���c�~
Precision oncology-����Q�m����Â̍őO���ɂ����鐬�ʂƖ��_�F�Q�m����Â̐V���Ȑi�W�Ɖۑ�@�S�Q�m����͂̎��n�Տ������̉ۑ�. ��ᇓ���, 34: 222-230, 2024
����R�L��
�����ȒP���. �����̗Տ�, 39: 252-253, 2024
����R�L��
�����̂��ׂ�2024�F�ŐV�̎��Á@���������ɑ���Ö@�@�ŐV�g�s�b�N�X�܂Ƃ�. ��w�̂����, 290: 422-426, 2024
����R�L��
�����w2024(��)-�ŐV�̐f�f�Ǝ���-�F�����̖Ö@�@���[������ї̈惊���p�ߍĔ������؏���̑S�g�Ö@�͂ǂ̂悤�ɍl���邩. ���{�Տ�, 82: 46-50, 2024
����R�L��
ICI��p�����e���킪��̕W���I���ÂƐV�K�Ɖu�Ö@�̉ۑ�F������ɂ����邪��Ɖu�Ö@. ��ᇓ���, 33: 108-115, 2024
����R�L��
������̍Ĕ��Ƃ͉���? ��ᇓ���, 34: 193-197, 2024
�t�R�D���b, �����G��, ���, �O�c�N��, �������q, ���숤��, �V��T��, ���q�i��, �����D��, �唗�q, �A�ؗL��, ���M�V, ���^�i
����`�q�p�l�������Őf�f�Ɏ�����Li-Fraumeni�nj�Q�̖����nj����ҁ@����̓��[�n�C���X�N�T�[�x�C�����X�ŗ��������̐f�f�Ɏ�����1��. ��`�����, 24: 153-160, 2024
�ؑ��D��
�ŐV�̐f�f�Ǝ��ÁF�o�C�I���W�[,��b�����̐i���@DNA�C���s�S�Ɠ���. ���{�Տ�, 82: 46-50, 2024
��앑��, �����v��, ����M�u, ��ؘj, ���a�X, ���ш�j, ���萒�l, �R��, ����������, �F�����, �[�c�ꕽ, ���엘��, �R�����a
�������p��FEC�Ö@�ɂ�����y�O�t�B���O���X�`���g�p�ɂ�錌�������̐��ڒ���. ���Ɖ��w�Ö@, 51: 913-918, 2024
�[�c�ꕽ, ���M�V
�킪���ɂ����邪��Q�m����Â̌��݂Ɩ����@�p�l����������S�Q�m����͌������܂߂�. ���{�O�Ȋw��G��, 125: 252-257, 2024
�[�c�ꕽ
���W�V��������̕��q��͋Z�p�̗Տ����p�F����S�Q�m����͂̍��Ƃ��ꂩ��. ���Ɖ��w�Ö@, 51: 7-13, 2024
�Љ�����, ��������, �A�O�ޒÌb, �g�c�މ�, ���i�L�I, �t�R�D���b, ������, �����j�q, ���L��, �R���ސ^, �g�c�a��, �O�c�N��, ���, ���ЕF, ��������, �_���b�q, ���엘��, �n粉�V, ���M�V
����f�Ë��_�a�@�Ƃ́@����f�Â̋ςĂ��l����@���ł��ǂ��ł��N����ł��T�o�C�o�[�V�b�v�P�A������a�@��ڎw����. ���{�O�Ȋw��G��, 125: 585-588, 2024
�Љ�����
�����w2024(��)-�ŐV�̐f�f�Ǝ���-�F�t�H���[�A�b�v�ƃg�[�^���w���X�P�A�@AYA��������̓����ƃT�o�C�o�[�V�b�v,�D�s���ێ�. ���{�Տ�, 82: 175-179, 2024
�Љ�����
AYA��������f��. ���{�Տ��O�Ȋw��G��, 85: 1-8, 2024
���엘��
G-CSF�K���g�p�K�C�h���C��2022�N��. ��ᇓ���, 33: 86-91, 2024
2023�N
- Yamauchi H, Toi M, Takayama S, Nakamura S, Takano T, Cui K, Campbell C, De Vos L, Geyer C, Jr., Tutt A.
Adjuvant olaparib in the subset of patients from Japan with BRCA1- or BRCA2-mutated high-risk early breast cancer from the phase 3 OlympiA trial. Breast Cancer 30: 696-605, 2023. - Ogiya A, Nagura N, Shimo A, Nogi H, Narui K, Seki H, Mori H, Sasada S, Ishitobi M, Kondo N, Yamauchi C, Akazawa K, Shien T.
ASO author reflections: Long term outcomes of breast cancer patients with local recurrence after mastectomy undergoing immediate breast reconstruction. Ann. Surg. Oncol. 30: 6541-6542, 2023. - Ogiya A, Nagura N, Shimo A, Nogi H, Narui K, Seki H, Mori H, Sasada S, Ishitobi M, Kondo N, Yamauchi C, Akazawa K, Shien T.
ASO visual abstract: Long-term outcomes of breast cancer patients with local recurrence after mastectomy undergoing immediate breast reconstruction: A retrospective, multi-institutional study of 4153 cases. Ann. Surg. Oncol. 30: 6453-6544, 2023. - Tada K, Kumamaru H, Miyata H, Asaga S, Iijima K, Ogo E, Kadoya T, Kubo M, Kojima Y, Tanakura K, Tamura K, Nagahashi M, Niikura N, Hayashi N, Miyashita M, Yoshida M, Ohno S, Imoto S, Jinno H.
Characteristics of female breast cancer in Japan: Annual report of the National Clinical Database in 2018. Breast Cancer 30: 157-166, 2023. - Takeda M, Kataoka A, Abe T, Inoue Y, Uehiro N, Takahashi Y, Nakashima E, Ogiya A, Sakai T, Morizono H, Miyagi Y, Ohno S, Ueno T.
Childbirth after perioperative systemic therapy in patients with breast cancer: A retrospective single institutional study in Japan. Jpn. J. Clin. Oncol. 53: 457-462, 2023. - Miyata K, Zhou X, Nishio M, Hanyu A, Chiba M, Kawasaki H, Osako T, Takeuchi K, Ohno S, Ueno T, Maruyama R, Takahashi A.
Chromatin conformational changes at human satellite II contribute to the senescence phenotype in the tumor microenvironment. Proc. Natl. Acad. Sci. U. S. A. 120: e2305046120, 2023. - Kumegawa K, Saeki S, Takahashi Y, Yang L, Osako T, Nakadai T, Amino S, Maeda T, Takahata C, Mori S, Noda T, Ohno S, Ueno T, Maruyama R.
Chromatin profile-based identification of a novel ER-positive breast cancer subgroup with reduced ER-responsive element accessibility. Br. J. Cancer. 128: 1208-1222, 2023. - Yamaguchi A, Ishitobi M, Nagura N, Shimo A, Seki H, Ogiya A, Sakurai T, Seto Y, Oshiro C, Sasada S, Kato M, Kawate T, Kondo N, Narui K, Nakagawa T, Nogi H, Yamauchi C, Tsugawa K, Kajiura Y, Shien T.
Classification of local recurrence after nipple-sparing mastectomy based on location: The features of nipple-areolar recurrence differ from those of other local recurrences. Ann. Surg. Oncol. 30: 1678-1686, 2023. - Baba T, Kusumoto M, Kato T, Kurihara Y, Sasaki S, Oikado K, Saito Y, Endo M, Fujiwara Y, Kenmotsu H, Sata M, Takano T, Kato K, Hirata K, Katagiri T, Saito H, Kuwano K.
Clinical and imaging features of interstitial lung disease in cancer patients treated with trastuzumab deruxtecan. Int. J. Clin. Oncol. 28: 1585-1596, 2023. - Shimomura A, Yoshida M, Kubo T, Yamashita S, Noguchi E, Nagayama A, Hanamura T, Okazaki M, Mukohara T, Tsuruga A, Tanaka K, Kawamura Y, Higuchi T, Takahashi Y, Kurozumi S, Hayashida T, Ichikawa H, Ushijima T, Suto A.
Clinicopathological features, genetic alterations, and BRCA1 promoter methylation in Japanese male patients with breast cancer. Breast Cancer Res. Treat. 197: 593-602, 2023. - Yamauchi H, Toi M, Takayama S, Nakamura S, Takano T, Cui K, Campbell C, De Vos L, Geyer C, Jr., Tutt A.
Correction: Adjuvant olaparib in the subset of patients from Japan with BRCA1- or BRCA2-mutated high-risk early breast cancer from the phase 3 OlympiA trial. Breast Cancer 30: 606, 2023. - Saeki S, Iwatani T, Kitano A, Sakurai N, Tanabe Y, Yamauchi C, Igarashi A, Kajimoto Y, Kuba S, Hara F, Sagara Y, Ohno S.
Correction: Factors associated with financial toxicity in patients with breast cancer in Japan: A comparison of patient and physician perspectives. Breast Cancer 30: 831, 2023. - Shimomura A, Takano T, Takahashi S, Sagara Y, Watanabe J, Tokunaga E, Shinkai T, Kamio T, Kikumori K, Kamiyama E, Fujisaki Y, Saotome D, Yamashita T.
Effect of trastuzumab deruxtecan on QT/QTc interval and pharmacokinetics in HER2-positive or HER2-low metastatic/unresectable breast cancer. Clin. Pharmacol. Ther. 113: 160-169, 2023. - Iwata H, Nakamura R, Masuda N, Yamashita T, Yamamoto Y, Kobayashi K, Tsurutani J, Iwasa T, Yonemori K, Tamura K, Aruga T, Tokunaga E, Kaneko K, Lee M J, Yuno A, Kawabata A, Seike T, Kaneda A, Nishimura Y, Trepel J B, Saji S.
Efficacy and exploratory biomarker analysis of entinostat plus exemestane in advanced or recurrent breast cancer: Phase II randomized controlled trial. Jpn. J. Clin. Oncol. 53: 4-15, 2023. - Takano T, Ito M, Kadoya T, Osako T, Aruga T, Masuda N, Miyaki T, Niikura N, Shimizu D, Yokoyama Y, Watanabe M, Tomomitsu M, Aogi K.
Efficacy and safety of pegfilgrastim biosimilar MD-110 in patients with breast cancer receiving chemotherapy: Single-arm phase III. Cancer Med. 12: 20242-20250, 2023. - Masuda H, Tanabe Y, Sakai H, Matsumoto K, Shimomura A, Doi M, Miyoshi Y, Takahashi M, Sagara Y, Tokunaga S, Iwasa T, Niikura N, Yoshimura K, Takano T, Tsurutani J.
Efficacy of probiotics and trimebutine maleate for abemaciclib-induced diarrhea: A randomized, open-label phase II trial (MERMAID, WJOG11318B). Breast 71: 22-28, 2023. - Masuda J, Sakai H, Tsurutani J, Tanabe Y, Masuda N, Iwasa T, Takahashi M, Futamura M, Matsumoto K, Aogi K, Iwata H, Hosonaga M, Mukohara T, Yoshimura K, Imamura C K, Miura S, Yamochi T, Kawabata H, Yasojima H, Tomioka N, Yoshimura K, Takano T.
Efficacy, safety, and biomarker analysis of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR-positive HER2-negative metastatic breast cancer: a phase II study (WJOG11418B NEWFLAME trial). J. Immunother. Cancer 11: e007126, 2023. - Yamamoto Y, Yamashiro H, Schneeweiss A, Müller V, Gluz O, Klare P, Aktas B, Magdolna D, Büdi L, Pikó B, Mangel L, Toi M, Morita S, Ohno S.
Factors affecting prognosis in patients treated with bevacizumab plus paclitaxel as first-line chemotherapy for HER2-negative metastatic breast cancer: An international pooled analysis of individual patient data from four prospective observational studies. Breast Cancer 30: 88-100, 2023. - Saeki S, Iwatani T, Kitano A, Sakurai N, Tanabe Y, Yamauchi C, Igarashi A, Kajimoto Y, Kuba S, Hara F, Sagara Y, Ohno S.
Factors associated with financial toxicity in patients with breast cancer in Japan: A comparison of patient and physician perspectives. Breast Cancer 30: 820-830, 2023. - Watanabe K, Niikura N, Kikawa Y, Oba M, Kobayashi K, Tada H, Ozaki S, Toh U, Yamamoto Y, Tsuneizumi M, Okuno T, Iwakuma N, Takeshita T, Iwamoto T, Ishiguro H, Masuda N, Saji S.
Fulvestrant plus palbociclib in advanced or metastatic hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer after fulvestrant monotherapy: Japan Breast Cancer Research Group-M07 (FUTURE trial). Breast Cancer Res. Treat. 199: 253-263, 2023. - Masuyama M, Masuda N, Kawaguchi H, Yamamoto Y, Saji S, Nakayama T, Aogi K, Anan K, Ohtani S, Sato N, Takano T, Tokunaga E, Nakamura S, Hasegawa Y, Hattori M, Fujisawa T, Morita S, Yamaguchi M, Yamashita T, Yotsumoto D, Toi M, Ohno S.
Fulvestrant with or without anti-HER2 therapy in patients in a postmenopausal hormonal state and with ER-positive HER2-positive advanced or metastatic breast cancer: A subgroup analysis of data from the Safari study (JBCRG-C06). Cancer Med. 12: 17718-17730, 2023. - Ozaki Y, Kinowaki K, Kawabata H, Kudo-Saito C.
IL25+ macrophages are a key determinant of treatment resistance of IL17RB+ breast cancer. Am. J. Cancer Res. 13: 4931-4943, 2023. - Ozaki Y, Masuda J, Kataoka A, Kogawa T, Abe T, Morizono H, Inagaki R, Hara F, Takano T, Ueno T, Ohno S.
The impact of obesity and endocrine therapy on the prognosis of premenopausal women with hormone receptor-positive breast cancer: A single-institute retrospective study. Cancer Rep. 6: e1695, 2023. - Nomura H, Abe A, Fusegi A, Yoshimitsu T, Misaka S, Murakami A, Matsumoto T, Tsumura S, Kanno M, Aoki Y, Netsu S, Omi M, Tanigawa T, Okamoto S, Omatsu K, Yunokawa M, Kanao H, Habano E, Arakawa H, Kaneko K, Ueki A, Haruyama Y, Inari H, Ueno T.
Impact of the coverage of risk-reducing salpingo-oophorectomy by the national insurance system for women with BRCA pathogenic variants in Japan. Sci. Rep. 13: 1018, 2023. - Nakayama I, Shinozaki E, Kawachi H, Sasaki T, Yunokawa M, Tomomatsu J, Yuasa T, Kitazono S, Kobayashi K, Hayakawa K, Ueki A, Takahashi S, Yamaguchi K.
Implementation of microsatellite instability testing for the assessment of solid tumors in clinical practice. Cancer Med. 12: 7932-7940, 2023. - Yokokawa T, Suzuki K, Tsuji D, Hosonaga M, Kobayashi K, Kawakami K, Kawazoe H, Nakamura T, Suzuki W, Sugisaki T, Aoyama T, Hashimoto K, Hatori M, Tomomatsu T, Inoue A, Azuma K, Asano M, Takano T, Ohno S, Yamaguchi M.
Influence of menopause on chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy for breast cancer: A retrospective observational study. Cancer Med. 12: 18745-18754, 2023. - Partridge A H, Niman S M, Ruggeri M, Peccatori F A, Azim H A, Jr., Colleoni M, Saura C, Shimizu C, Sætersdal A B, Kroep J R, Mailliez A, Warner E, Borges V F, Amant F, Gombos A, Kataoka A, Rousset-Jablonski C, Borstnar S, Takei J, Lee J E, Walshe J M, Ruíz-Borrego M, Moore H C F, Saunders C, Bjelic-Radisic V, Susnjar S, Cardoso F, Smith K L, Ferreiro T, Ribi K, Ruddy K, Kammler R, El-Abed S, Viale G, Piccart M, Korde L A, Goldhirsch A, Gelber R D, Pagani O.
Interrupting endocrine therapy to attempt pregnancy after breast cancer. N. Engl. J. Med. 388: 1645-1656, 2023. - Teruya N, Inoue H, Horii R, Akiyama F, Ueno T, Ohno S, Takahashi S.
Intratumoral heterogeneity, treatment response, and survival outcome of ER-positive HER2-positive breast cancer. Cancer Med. 12: 10526-10535, 2023. - Terada M, Ito A, Kikawa Y, Koizumi K, Naito Y, Shimoi T, Ishihara M, Yamanaka T, Ozaki Y, Hara F, Nakamura R, Hattori M, Miyashita M, Kondo N, Yoshinami T, Takada M, Matsumoto K, Narui K, Sasada S, Iwamoto T, Hosoda M, Takano Y, Oba T, Sakai H, Murakami A, Higuchi T, Tsuchida J, Tanabe Y, Shigechi T, Tokuda E, Harao M, Kashiwagi S, Mase J, Watanabe J, Nagai S E, Yamauchi C, Yamamoto Y, Iwata H, Saji S, Toyama T.
The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2022 edition. Breast Cancer 30: 872-884, 2023. - Ogiya A, Nagura N, Shimo A, Nogi H, Narui K, Seki H, Mori H, Sasada S, Ishitobi M, Kondo N, Yamauchi C, Akazawa K, Shien T.
Long-term outcomes of breast cancer patients with local recurrence after mastectomy undergoing immediate breast reconstruction: A retrospective multi-institutional study of 4153 cases. Ann. Surg. Oncol. 30: 6532-6540, 2023. - Ueno T.
A message from the new editor-in-chief. Breast Cancer 30: 1, 2023. - Ishiba T, Nishibuchi I, Hara F, Shikama N, Shien T, Iwata H.
Metastasis-directed therapy for oligometastases in breast cancer. Jpn. J. Clin. Oncol. 53: 893-898, 2023. - Funasaka C, Hanai A, Zenda S, Mori K, Fukui M, Hirano N, Shinohara R, Fuse N, Wakabayashi M, Itagaki M, Tomioka Y, Nishina M, Arai Y, Kogawa T, Ozaki Y, Nishimura M, Kobayashi T, Hara F, Takano T, Mukohara T.
Mitigation of paclitaxel-induced peripheral neuropathy in breast cancer patients using limb-cooling apparatus: A study protocol for a randomized controlled trial. Front. Oncol. 13: 1216813, 2023. - Sakai H, Tsurutani J, Ozaki Y, Ishiguro H, Nozawa K, Watanabe K, Maeda S, Yokoe T, Imamura C K, Matsumoto K, Iwasa T, Chiba Y, Takiguchi D, Takano T.
Multicentre, randomised, double-blind, placebo-controlled phase II study of prophylactic olanzapine for patients with metastatic breast cancer receiving T-DXd treatment: protocol for the ERICA study (WJOG14320B). BMJ Open 13: e070304, 2023. - Koizumi T, Sugishita Y, Suzuki-Takahashi Y, Nara K, Miyagawa T, Nakajima M, Sugimoto K, Futamura M, Furui T, Takai Y, Matsumoto H, Yamauchi H, Ohno S, Kataoka A, Kawai K, Fukuma E, Nogi H, Tsugawa K, Suzuki N.
Oncofertility-related psycho-educational therapy for young adult patients with breast cancer and their partners: Randomized controlled trial. Cancer 129: 2568-2580, 2023. - Hattori M, Masuda N, Takano T, Tsugawa K, Inoue K, Matsumoto K, Ishikawa T, Itoh M, Yasojima H, Tanabe Y, Yamamoto K, Suzuki M, Pan W, Cortes J, Iwata H.
Pembrolizumab plus chemotherapy in Japanese patients with triple-negative breast cancer: Results from KEYNOTE-355. Cancer Med. 12: 10280-10293, 2023. - Kobayashi K, Masuda N, Mizuno T, Miura K, Tokuda Y, Yoshinami T, Kawaguchi H, Ohtani S, Saeki T, Toi M, Takeuchi M, Ito Y.
Phase II trial of biweekly administration with eribulin after three cycles of induction therapy in hormone receptor-positive, HER2-negative metastatic breast cancer (JACCRO BC-03). Breast Cancer Res. Treat. 201: 409-415, 2023. - Iwata H, Yamamoto Y, Sakai T, Hasegawa Y, Nakamura R, Akabane H, Ohtani S, Kashiwaba M, Taira N, Toyama T, Fujisawa T, Masuda N, Shibahara Y, Sasano H, Yamaguchi T.
Phase III study of long-term prognosis of estrogen receptor-positive early breast cancer treated with neoadjuvant endocrine therapy with/without adjuvant chemotherapy. Breast Cancer Res. Treat. 199: 231-241, 2023. - Yamakado R, Ishitobi M, Kondo N, Yamauchi C, Sasada S, Nogi H, Saiga M, Ogiya A, Narui K, Seki H, Nagura N, Shimo A, Sakurai T, Niikura N, Mori H, Shien T.
Physicians' perception about the impact of breast reconstruction on patient prognosis: A survey in Japan. Breast Cancer 30: 302-308, 2023. - Iwase T, Saji S, Iijima K, Higaki K, Ohtani S, Sato Y, Hozumi Y, Hasegawa Y, Yanagita Y, Takei H, Tanaka M, Masuoka H, Tanabe M, Egawa C, Komoike Y, Nakamura T, Ohtsu H, Mukai H.
Postoperative adjuvant anastrozole for 10 or 5 years in patients with hormone receptor-positive breast cancer: AERAS, a randomized multicenter open-label phase III trial. J. Clin. Oncol. 41: 3329-3338, 2023. - �[�c�ꕽ, ���M�V.
Precision oncology�̌��F����S�Q�m����̗͂Տ����p�T�v�F���ҊҌ��ǂ̖ړI�Ɖۑ�. ��ᇓ��� 31: 8-15, 2023. - Aoki Y, Inoue Y, Sasahira N, Ono M, Inamura K, Kataoka A, Takano T, Kanao H, Watanabe M.
Primary ovarian insufficiency associated with lenvatinib therapy in a patient with hepatocellular carcinoma: A case report. Oncol. Lett. 26: 450, 2023. - Sasada S, Kondo N, Hashimoto H, Takahashi Y, Terata K, Kida K, Sagara Y, Ueno T, Anan K, Suto A, Kanbayashi C, Takahashi M, Nakamura R, Ishiba T, Tsuneizumi M, Nishimura S, Naito Y, Hara F, Shien T, Iwata H.
Prognostic impact of adjuvant endocrine therapy for estrogen receptor-positive and HER2-negative T1a/bN0M0 breast cancer. Breast Cancer Res. Treat. 202: 473-483, 2023. - Fukada I, Mori S, Hayashi N, Hosonaga M, Xiaofei W, Yamazaki M, Ueki A, Kiyotani K, Tonooka A, Takeuchi K, Ueno T, Takahashi S.
Prognostic impact of cancer genomic profile testing for advanced or metastatic solid tumors in clinical practice. Cancer Sci. 114: 4632-4642, 2023. - Shibata N, Yoshinami T, Tamaki K, Nukada T, Ohno S.
Real-world data analysis of perioperative chemotherapy patterns, G-CSF use, and FN status in patients with early breast cancer. Breast Cancer Res. Treat. 201: 265-273, 2023. - Nozawa K, Terada M, Onishi M, Ozaki Y, Takano T, Fakhouri W, Novick D, Haro J M, Faris L H, Kawaguchi T, Tanizawa Y, Tsurutani J.
Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR + , HER2- metastatic breast cancer patients in Japan. Breast Cancer 30: 657-665, 2023. - Kawaguchi H, Yamamoto Y, Saji S, Masuda N, Nakayama T, Aogi K, Anan K, Ohtani S, Sato N, Takano T, Tokunaga E, Nakamura S, Hasegawa Y, Hattori M, Fujisawa T, Morita S, Yamaguchi M, Yamashita T, Yotsumoto D, Toi M, Ohno S.
Retrospective study on the effectiveness of medroxyprogesterone acetate in the treatment of ER-positive/HER2-negative post-menopausal advanced breast cancer: An additional analysis of the JBCRG-C06 Safari study. Jpn. J. Clin. Oncol. 53: 203-211, 2023. - Takada M, Imoto S, Ishida T, Ito Y, Iwata H, Masuda N, Mukai H, Saji S, Ikeda T, Haga H, Saeki T, Aogi K, Sugie T, Ueno T, Ohno S, Ishiguro H, Kanbayashi C, Miyamoto T, Hagiwara Y, Toi M.
A risk-based subgroup analysis of the effect of adjuvant S-1 in estrogen receptor-positive, HER2-negative early breast cancer. Breast Cancer Res. Treat. 202: 485-496, 2023. - Abe A, Nomura H, Fusegi A, Yunokawa M, Ueki A, Habano E, Arakawa H, Kaneko K, Minoura Y, Inari H, Ueno T, Kanao H.
Risk-reducing decisions regarding germline BRCA pathogenic variant: Focusing on the timing of genetic testing and RRSO. J .Med. Genet. in press, 2023. - Akechi T, Yamaguchi T, Uchida M, Imai F, Momino K, Katsuki F, Sakurai N, Miyaji T, Mashiko T, Horikoshi M, Furukawa T A, Yoshimura A, Ohno S, Uehiro N, Higaki K, Hasegawa Y, Akahane K, Uchitomi Y, Iwata H.
Smartphone psychotherapy reduces fear of cancer recurrence among breast cancer survivors: A fully decentralized randomized controlled clinical trial (J-SUPPORT 1703 Study). J. Clin. Oncol. 41: 1069-1078, 2023. - Imoto S, Wang K, Bi X W, Liu G, Im Y H, Im S A, Sim S H, Ueno T, Futamura M, Toi M, Fujiwara Y, Ahn S G, Lee J E, Park Y H, Takao S, Oba M S, Kitagawa Y, Nishiyama M.
Survival advantage of locoregional and systemic therapy in oligometastatic breast cancer: An international retrospective cohort study (OLIGO-BC1). Breast Cancer 30: 412-423, 2023. - Saeki S, Kumegawa K, Takahashi Y, Yang L, Osako T, Yasen M, Otsuji K, Miyata K, Yamakawa K, Suzuka J, Sakimoto Y, Ozaki Y, Takano T, Sano T, Noda T, Ohno S, Yao R, Ueno T, Maruyama R.
Transcriptomic intratumor heterogeneity of breast cancer patient-derived organoids may reflect the unique biological features of the tumor of origin. Breast Cancer Res. 25: 21, 2023. - André F, Hee Park Y, Kim S B, Takano T, Im S A, Borges G, Lima J P, Aksoy S, Gavila Gregori J, De Laurentiis M, Bianchini G, Roylance R, Miyoshi Y, Armstrong A, Sinha R, Ruiz Borrego M, Lim E, Ettl J, Yerushalmi R, Zagouri F, Duhoux F P, Fehm T, Gambhire D, Cathcart J, Wu C, Chu C, Egorov A, Krop I.
Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 401: 1773-1785, 2023. - �R�z��.
���҂̃A�s�A�����X�P�A. ��ᇓ��� 32: 195-200, 2023. - ���c�从q, ���엘��, �������K, ���c���K, �������K, ���ΐ���Y.
���҂̎d���Ǝ��Â̗����x��. ��ᇓ��� 32: 189-194, 2023. - ���^�i.
����f�Âɂ�����w�ʉ���Â̌���ƍ���̓W�]�F������ɂ�����w�ʉ���Â̌���ƍ���̓W�]. ���{�O�Ȋw��G�� 124: 239-245, 2023. - �R�z��, ����R�L��, ���엘��.
���ꂾ���͉������Ă��������F���w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g�F����������!�x���Ö@��G���M���D���������ǂł̎x���Ö@��. YORi-SOU����i�[�V���O �ʍ�: 366-368, 2023. - ���M�V.
���ꂾ���͉������Ă��������F���w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g�F���q�W�I��F�RHER2�R�́F�g���X�c�Y�}�u�G���^���V��. YORi-SOU����i�[�V���O �ʍ�: 34-35, 2023. - ���M�V.
���ꂾ���͉������Ă��������F���w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g�F���q�W�I��F�RHER2�R�́F�g���X�c�Y�}�u�f���N�X�e�J��. YORi-SOU����i�[�V���O �ʍ�: 32-33, 2023. - ���M�V.
���ꂾ���͉������Ă��������F���w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g�F���q�W�I��F�RHER2�R�́F�y���c�Y�}�u. YORi-SOU����i�[�V���O �ʍ�: 36-37, 2023. - ����R�L��.
������f�Â̐V�����i�F�FctDNA���猩���������f�Â̐V�����i�F. ��ᇓ��� 32: 123-128, 2023. - �Љ�����, ��������, ���^�i.
�������҂̔D�s��������POSITIVE�����̈Ӌ`. ��ᇓ��� 32: 166-170, 2023. - ���M�V.
�����f�f�Ǝ��Âɂ����鉟������ׂ��g�����h�FADC�o�ꂪ�����炵���V���ȓ����T�u�^�C�vHER2-low(HER2�ᔭ��). Rad Fan 21: 49-51, 2023. - ����R�L��.
�����f�f�Ǝ��Âɂ����鉟������ׂ��g�����h�F�����ɂ�����ctDNA�̈ʒu�t��. Rad Fan 21: 59-63, 2023. - �Љ�����, ��������, ���^�i.
���B�F�Y�w�l�Ȉオ�m���Ă����ׂ��K�{�m���F������̑��������F�z��������[�Ö@,�s���Ɠ����X�N�E���f�̗L����. �Տ��w�l�ȎY�� 77: 384-388, 2023. - ���i�L�I, �Љ�����.
���֓����̎����F���֓����̔���,��U�Ǝ���. �`���O�� 66: 529-537, 2023. - �i�^��, ���엘��.
�e���킪��ɑ�����p��(��p�E���ː�����)�Ö@��State of the art�F������. ��ᇓ��� 32: 230-236, 2023. - ����R�L��, ���엘��.
���ۋ��������ւ̉ۑ�F���w�Ö@�ɂ�����A�W�A�Ɖ��Ă̈Ⴂ�F���ۋ��������Ə��F�i���ւ̉ۑ�. ���Ɖ��w�Ö@ 50: 30-34, 2023. - ������, �{��R��, �����m�q, ���, ���J�N�q, �Љ�����, ���ЕF, ���M�V, �唗�q, ���^�i.
�َ����ɗ������[�ɔF�߂����Ǔ���ɑ��ĊO�ȓI�؏����L�p�ł��������. �����̗Տ� 38: 281-286, 2023. - �R�z��, ���엘��.
�Ǘ�Ɋw�ԗՏ������f�[�^�̐f�����F�̈�ʗՏ������f�[�^�̐f�����F��ᇃ}�[�J�[. ��ଂƌ��� 100: 229-233, 2023. - �ܖ�����, �[�c�ꕽ, ������L, �����r��.
�Ǘጟ����F������Ö@��R����IV���̐Z�������t���F�G�L�X�p�[�g�p�l���ł̌����܂���. Cancer Borad Breast 7: 71-80, 2023. - ���J�N�q, �ؑ�����, �␣��m, ���M�V, ���^�i.
�����|�s���C�Z���`�l�������p�ߐ�����̌o�N�I�ȃ����p����̐��ڂƕ�����֘A���q. ���{�����p����w��G�� 4: 10-14, 2023. - ���ЕF.
�p�O���w�Ö@���{�s����cN �����ɂ��������|����-�e�{�݂̎��g�݁F�p�O���w�Ö@���t���������|�����p�ߓ]�ڂ�L��������ɑ������|��p�ɂ��āF���L���a�@�ɂ�����O�����ώ@�����̏Љ�. �����̗Տ� 38: 175-180, 2023.
2022�N
- Sawaki M, Taira N, Uemura Y, Saito T, Baba S, Kobayashi K, Kawashima H, Tsuneizumi M, Sagawa N, Bando H, Takahashi M, Yamaguchi M, Takashima T, Nakayama T, Kashiwaba M, Mizuno T, Yamamoto Y, Iwata H, Toyama T, Tsugawa K, Kawahara T, Mukai H.
Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: A propensity score-adjusted analysis of a prospective cohort study. Breast 66: 245-254, 2022. - Oshiro R, Soejima T, Tada K, Suzuki M, Ohno S, Yubune K, Nakamura S, Fukuchimoto H, Takei J, Yamauchi H, Kamibeppu K.
Anxiety and related factors among parents of patients with breast cancer after surgery in Japan: A multi-informant and multilevel study. Jpn. J. Nurs. Sci. 19: e12452, 2022. - Fukada I, Mori S, Hayashi N, Hosonaga M, Yamazaki M, Wang X, Kawai S, Inagaki L, Ozaki Y, Kobayashi K, Hara F, Kobayashi T, Ueki A, Osako T, Tonooka A, Takeuchi K, Ueno T, Takano T, Ohno S, Takahashi S.
Assessment of a cancer genomic profile test for patients with metastatic breast cancer. Sci. Rep. 12: 4813, 2022. - Razis E, Escudero M J, Palmieri C, Mueller V, Bartsch R, Rossi G, Gampenrieder S P, Kolberg H C, Zdenkowski N, Pavic M, Connolly R M, Rosset L, Arcuri J, Tesch H, Vallejos C, Retamales J, Musolino A, Del Mastro L, Christodoulou C, Aebi S, Paluch-Shimon S, Gupta S, Ohno S, Macpherson I, Ekholm M, Zaman K, Vidal M, Chakiba C, Fumagalli D, Thulin A, Witzel I, Kotecki N, Gil-Gil M, Linderholm B.
Assessment of the management of carcinomatous meningitis from breast cancer globally: A study by the Breast International Group Brain Metastasis Task Force. ESMO Open 7: 100483, 2022. - Abe Y, Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F, Shien T, Doihara H, Toyooka S.
Association of genetic polymorphism with taxane-induced peripheral neuropathy: Sub-analysis of a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in metastatic breast cancer patients. Acta Med. Okayama 76: 661-671, 2022. - Kataoka A, Ueno T, Yamauchi H, Uehiro N, Takahata C, Takahashi Y, Nakashima E, Ogiya A, Sakai T, Kitagawa D, Morizono H, Miyagi Y, Iwase T, Kitano A, Fukatsu Y, Tamura N, Kawano J, Bando H, Tamaki K, Shiota K, Ozawa M, Kobayashi M, Ohno S.
Characteristics, treatment trends, and long-term outcomes of Japanese patients with pregnancy-associated breast cancer (PABC). Breast Cancer 29: 825-834, 2022. - Inoue Y, Fujishima M, Ono M, Masuda J, Ozaki Y, Maeda T, Uehiro N, Takahashi Y, Kobayashi T, Sakai T, Osako T, Ueno T, Ohno S.
Clinical significance of the neutrophil-to-lymphocyte ratio in oligometastatic breast cancer. Breast Cancer Res. Treat. 196: 341-348, 2022. - Aogi K, Watanabe K, Kitada M, Sangai T, Ohtani S, Aruga T, Kawaguchi H, Fujisawa T, Maeda S, Morimoto T, Sato N, Takao S, Morita S, Masuda N, Toi M, Ohno S.
Correction to: Clinical usefulness of eribulin as first- or second-line chemotherapy for recurrent HER2-negative breast cancer: A randomized phase II study (JBCRG-19). Int. J. Clin. Oncol. 27: 1793, 2022. - Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F.
Correction to: Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast Cancer 29: 186-188, 2022. - Tsuda H, Kurosumi M, Akiyama F, Ohno S, Saji S, Masuda N, Shimomura A, Sato N, Takao S, Ohsumi S, Tokuda Y, Inaji H, Watanabe T.
Correction to: Validation of a nuclear grading system for resected stage I-IIIA, high-risk, node-negative invasive breast carcinoma in the N·SAS-BC 01 trial. Breast Cancer 29: 730, 2022. - Takumoto Y, Shiroiwa T, Shimozuma K, Iwata H, Takahashi M, Baba S, Kobayashi K, Hagiwara Y, Kawahara T, Uemura Y, Mukai H, Taira N, Sawaki M.
Cost-effectiveness of trastuzumab with or without chemotherapy as adjuvant therapy in HER2-positive elderly breast cancer patients: A randomized, open-label clinical trial, the RESPECT trial. Clin. Drug Investig. 42: 253-262, 2022. - Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T.
Data of programmed death-ligand 1 expression and VEGF: Nivolumab, bevacizumab and paclitaxel for HER2-negative. Data Brief 45: 108558, 2022. - Wang X, Kaneko K, Arakawa H, Habano E, Omi M, Nakashima E, Kawachi H, Tonooka A, Omatsu K, Nomura H, Yunokawa M, Kanao H, Takahashi S, Nakajima T, Ueki A.
Detection of BRCA1 pathogenic variant in a 24-year-old endometrial cancer patient: Risks of several hereditary tumor syndromes assessed by germline multigene panel testing. Case. Rep. Oncol. 15: 792-797, 2022. - Ithimakin S, Parinyanitikul N, Kim S B, Yap Y S, Tsang J, Soong I S, Ozaki Y, Ohno S, Ono M, Chan J J, Cheng H C S, Dejthevaporn T.
Disparities in access to systemic treatment for breast cancer in Thailand and major Asian territories. J. Breast Cancer 25: 207-217, 2022. - Ohmoto A, Hayashi N, Fukada I, Yamazaki M, Yunokawa M, Kasuga A, Shinozaki E, Ueki A, Tonooka A, Takeuchi K, Mori S, Kiyotani K, Takahashi S.
Druggable gene alterations in Japanese patients with rare malignancy. Neoplasia 33: 100834, 2022. - Fukuoka M, Ichikawa Y, Osako T, Fujita T, Baba S, Takeuchi K, Tsunoda N, Ebata T, Ueno T, Ohno S, Saitoh N.
The ELEANOR noncoding RNA expression contributes to cancer dormancy and predicts late recurrence of estrogen receptor-positive breast cancer. Cancer Sci. 113: 2336-2351, 2022. - Hayashi N, Fukada I, Ohmoto A, Yamazaki M, Wang X, Hosonaga M, Takahashi S.
Evaluation of an inflammation-based score for identification of appropriate patients for comprehensive genomic profiling. Discov. Oncol. 13: 109, 2022. - Kawaguchi H, Yamamoto Y, Saji S, Masuda N, Nakayama T, Aogi K, Anan K, Ito Y, Ohtani S, Sato N, Takano T, Tokunaga E, Nakamura S, Hasegawa Y, Hattori M, Fujisawa T, Morita S, Yamaguchi M, Yamashita H, Yamashita T, Yotsumoto D, Toi M, Ohno S.
Factors associated with overall survival after recurrence in patients with ER-positive/HER2-negative postmenopausal breast cancer: An ad hoc analysis of the JBCRG-C06 Safari study. Jpn. J. Clin. Oncol. 52: 545-553, 2022. - �t�R�D���b, ���q�i��, ���, ������, �O�c�N��, �����G��, �i�^��, ���ї��V, ���엘��, ���M�V, �A�ؗL��, ���^�i.
Gene awareness. ��ᇓ��� 30: 199-203, 2022. - Kumegawa K, Takahashi Y, Saeki S, Yang L, Nakadai T, Osako T, Mori S, Noda T, Ohno S, Ueno T, Maruyama R.
GRHL2 motif is associated with intratumor heterogeneity of cis-regulatory elements in luminal breast cancer. NPJ Breast Cancer 8: 70, 2022. - �ܖ�����, ������L, �e�r�^��, �����G��.
HBOC(��`�������������nj�Q)�ɑ���MRI�T�[�x�C�����X�Ŕ������ꂽ������1��. Cancer Borad Breast 7: 5-16, 2022. - �V��T��, ���q�i��, ���숤��, �쑽����, ���䏠�q, �t�R�D���b, �O�c�N��, �����G��, ���, ���M�V, �A�ؗL��, ���^�i.
HBOC�����Ǖψٕێ��҂̓����f�ƃT�|�[�g�̐��F���@�ł̂�����BRCA1/2�a�I�o���A���g�ێ��҂ւ̑Ή��o���Ƃ��ꂩ��̉ۑ�. ���{�������f�w� 31: 163-170, 2022. - Ueno T, Kitano S, Masuda N, Ikarashi D, Yamashita M, Chiba T, Kadoya T, Bando H, Yamanaka T, Ohtani S, Nagai S, Nakayama T, Takahashi M, Saji S, Aogi K, Velaga R, Kawaguchi K, Morita S, Haga H, Ohno S, Toi M.
Immune microenvironment, homologous recombination deficiency, and therapeutic response to neoadjuvant chemotherapy in triple-negative breast cancer: Japan Breast Cancer Research Group (JBCRG)22 TR. BMC Med. 20: 136, 2022. - Tokunaga E, Masuda T, Ijichi H, Tajiri W, Koga C, Koi Y, Nakamura Y, Ohno S, Taguchi K, Okamoto M.
Impact of serum vitamin D on the response and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer 29: 156-163, 2022. - Matsui T, Iwasa A, Mimura M, Taniguchi S, Sudo T, Uchida Y, Kikuta J, Morizono H, Horii R, Motoyama Y, Morii E, Ohno S, Kiyota Y, Ishii M.
Label-free multiphoton excitation imaging as a promising diagnostic tool for breast cancer. Cancer Sci. 113: 2916-2925, 2022. - Kosaka Y, Saeki T, Takano T, Aruga T, Yamashita T, Masuda N, Koibuchi Y, Osaki A, Watanabe J, Suzuki R.
Multicenter randomized open-label phase II clinical study comparing outcomes of NK105 and paclitaxel in advanced or recurrent breast cancer. Int. J. Nanomedicine 17: 4567-4578, 2022. - Yamamoto Y, Iwata H, Taira N, Masuda N, Takahashi M, Yoshinami T, Ueno T, Toyama T, Yamanaka T, Takano T, Kashiwaba M, Tsugawa K, Hasegawa Y, Tamura K, Tada H, Hara F, Fujisawa T, Niikura N, Saji S, Morita S, Toi M, Ohno S.
Pertuzumab retreatment for HER2-positive advanced breast cancer: A randomized, open-label phase III study (PRECIOUS). Cancer Sci. 113: 3169-3179, 2022. - Masuda N, Ono M, Mukohara T, Yasojima H, Shimoi T, Kobayashi K, Harano K, Mizutani M, Tanioka M, Takahashi S, Kogawa T, Suzuki T, Okumura S, Takase T, Nagai R, Semba T, Zhao Z M, Ren M, Yonemori K.
Phase 1 study of the liposomal formulation of eribulin (E7389-LF): Results from the breast cancer expansion cohort. Eur. J. Cancer 168: 108-118, 2022. - Yamada A, Hayashi N, Kumamaru H, Nagahashi M, Usune S, Asaga S, Iijima K, Kadoya T, Kojima Y, Kubo M, Miyashita M, Miyata H, Ogo E, Tamura K, Tanakura K, Tada K, Niikura N, Yoshida M, Ohno S, Ishikawa T, Narui K, Endo I, Imoto S, Jinno H.
Prognostic impact of postoperative radiotherapy in patients with breast cancer and with pT1-2 and 1-3 lymph node metastases: A retrospective cohort study based on the Japanese Breast Cancer Registry. Eur. J. Cancer 172: 31-40, 2022. - Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F.
Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast Cancer 29: 131-143, 2022. - Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T.
Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: Primary results and biomarker data from a phase 2 trial (WJOG9917B). Eur. J. Cancer 171: 193-202, 2022. - Chin Y M, Shibayama T, Chan H T, Otaki M, Hara F, Kobayashi T, Kobayashi K, Hosonaga M, Fukada I, Inagaki L, Ono M, Ito Y, Takahashi S, Ohno S, Ueno T, Nakamura Y, Low S K.
Serial circulating tumor DNA monitoring of CDK4/6 inhibitors response in metastatic breast cancer. Cancer Sci. 113: 1808-1820, 2022. - Saji S, Ohsumi S, Ito M, Hayashi N, Kobayashi K, Masuda N, Niikura N, Yamashita T, Kiyama K, Hasegawa A, Nakagawa S, Hattori M.
Subgroup analysis of Japanese patients in a phase III randomized, controlled study of neoadjuvant atezolizumab or placebo, combined with nab-paclitaxel and anthracycline-based chemotherapy in early triple-negative breast cancer (IMpassion031). Jpn. J. Clin. Oncol. 52: 1124-1133, 2022. - Ueno T.
Surgical management of metastatic breast cancer: A mini review. Front. Oncol. 12: 910544, 2022. - Saji S, Taira N, Kitada M, Takano T, Takada M, Ohtake T, Toyama T, Kikawa Y, Hasegawa Y, Fujisawa T, Kashiwaba M, Ishida T, Nakamura R, Yamamoto Y, Toh U, Iwata H, Masuda N, Morita S, Ohno S, Toi M.
Switch maintenance endocrine therapy plus bevacizumab after bevacizumab plus paclitaxel in advanced or metastatic oestrogen receptor-positive, HER2-negative breast cancer (BOOSTER): A randomised, open-label, phase 2 trial. Lancet Oncol. 23: 636-649, 2022. - Ozaki Y, Aoyama Y, Masuda J, Inagaki L, Kawai S, Shibayama T, Maeda T, Kurata M, Yoshida K, Saeki S, Hosonaga M, Fukada I, Hara F, Kobayashi T, Kobayashi K, Miyake S, Takano T, Ueno T, Ohno S.
Trastuzumab and fulvestrant combination therapy for women with advanced breast cancer positive for hormone receptor and human epidermal growth factor receptor 2: A retrospective single-center study. BMC Cancer 22: 36, 2022. - Jerusalem G, Park Y H, Hurvitz S A, Modi S, Andre F, Krop I E, Gonzalez-Farre X, You B, Saura C, Kim S B, Osborne C R, Murthy R K, Gianni L, Takano T, Liu Y, Cathcart J, Lee C, Perrin C.
Trastuzumab deruxtecan in HER2-positive metastatic breast cancer patients with brain metastases: A DESTINY-breast01 subgroup analysis. Cancer Discov. 12: 2754-2762, 2022. - Krop I E, Im S A, Barrios C, Bonnefoi H, Gralow J, Toi M, Ellis P A, Gianni L, Swain S M, Im Y H, De Laurentiis M, Nowecki Z, Huang C S, Fehrenbacher L, Ito Y, Shah J, Boulet T, Liu H, Macharia H, Trask P, Song C, Winer E P, Harbeck N.
Trastuzumab emtansine plus pertuzumab versus taxane plus trastuzumab plus pertuzumab after anthracycline for high-risk human epidermal growth factor receptor 2-positive early breast cancer: The phase III KAITLIN study. J. Clin. Oncol. 40: 438-448, 2022. - Tsuda H, Kurosumi M, Akiyama F, Ohno S, Saji S, Masuda N, Shimomura A, Sato N, Takao S, Ohsumi S, Tokuda Y, Inaji H, Watanabe T.
Validation of a nuclear grading system for resected stage I-IIIA, high-risk, node-negative invasive breast carcinoma in the N·SAS-BC 01 trial. Breast Cancer 29: 720-729, 2022. - ������.
����Q�m����Î���̕��q��ᇊw�F(��2��)��ᇔ����ɂ�����镪�q�@�\�FPI3K/AKT/mTOR�o�H. �a���ƗՏ� 40: 096-100, 2022. - �Љ�����, ��������, ���^�i.
����ȂƂ��ǂ�����?���ȂƂ̃R�~���j�P�[�V�����K�C�h�F(��13��)���B�O�ȁF�z��������[�Ö@�L�Q���ۂƂ��Ă̓�����. �Y�Ȃƕw�l�� 89: 463-467, 2022. - ���엘��.
�Z���t�P�A�x���E���n�r���e�[�V�����E�S���I�T�|�[�g�F���E�킾����ł��������Ō�g�[�^���P�A�F�͂��߂ɁF�Ō�t�ɓ`�������I������Ö@�̂��܂ƍŐV�g�s�b�N�X. YORi-SOU����i�[�V���O 12: 422-425, 2022. - �������q.
������f�Â̐i���ƓW�]�FCDK4/6�j�Q��ϐ����@���Ƃ��̍���. ��ᇓ��� 30: 150-156, 2022. - ��������, �Љ�����, ���^�i.
������f�Â̐i���ƓW�]�F�D�s�������Ɠ����Èオ�ł��邱��. ��ᇓ��� 30: 186-192, 2022. - ����R�L��, ���L��, ���^�i.
�����f�Â̍ŐV�̒m���F���ꂩ��̓����f��. ��ଂƌ��� 99: 1006-1010, 2022. - ���M�V.
�ŐV�E�]�ڐ��̎�ᇂ̊O�Ȏ��Âƈ�`�q�����̎��Âւ̍l�����F���B�E�w�l�Ȋ��F�������E�����̓]�ڂ̎��Âƈ�`�q����. �̑��N���j�J���A�b�v�f�[�g 8: 179-184, 2022. - ���V�r��, �㐙��], ���D�M, �ݗm��, �c�ߔE, ���^�i.
�O���A�g�͊�̌�����W�ƕԏ�����. �a�@���j�� 11-16, 2022. - �������q, ������.
����`�q������̗͂Տ����p�FOncotype DX. �����̗Տ� 37: 369-376, 2022. - ��F�z�q, �ؗm��, �����T��, ���얃�I�q, �|�����o�q, ���䖃���, �㓡�u��, �Љ�����, ���c���q, ���R��q, �R�c��q, ��Ȍd��, �s����, ���엘��, �n粉�V.
�D�s�������Ɋւ��邪����L���a�@��Ï]���҂̈ӎ������Ɠ��@�̌���. ���{����E���B��Êw� 5: 48-54, 2022. - �M�u���I, �x�䗝�G, �Ɖ��Ȃ�, ���M�V, ���^�i.
�����Z���������̗\��ƗՏ��a���w�I����. ���{�Տ��O�Ȋw��G�� 83: 12-18, 2022. - ��������, �Љ�����, ���J�N�q, �A�O�ޒÌb, �t�R�D���b, ���쌒��, ������, ������, �����G��, �����j�q, ���L��, �g�c�a��, �O�c�N��, �����m�q, ���, ���ЕF, �{��R��, ���M�V, ���^�i.
�j�����킸�O�Ȉオ�P�������邽�߂ɁF�n�C�{�����[���Z���^�[�ɂ����鎝���\�ȓ��������v. ���{�O�Ȋw��G�� 123: 486, 2022.
2021�N
- ���ї��V, ���^�i.
10�N��̂���f�ÁF10�N��̓�����f�Â��������āA�����g�ނׂ����T�[�`�j�[�Y. ��ᇓ��� 28: 216-228, 2021. - �[�c�ꕽ.
10�N��̂���f�ÁF�킪���ɂ�����Q�m����Â̌���ƓW�]. ��ᇓ��� 28: 262-268, 2021. - Toi M, Imoto S, Ishida T, Ito Y, Iwata H, Masuda N, Mukai H, Saji S, Shimizu A, Ikeda T, Haga H, Saeki T, Aogi K, Sugie T, Ueno T, Kinoshita T, Kai Y, Kitada M, Sato Y, Jimbo K, Sato N, Ishiguro H, Takada M, Ohashi Y, Ohno S.
Adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive, HER2-negative, primary breast cancer: A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22: 74-84, 2021. - �������F, ��؈�m, ���앐, ���^�i.
AI�z�X�s�^���ɂ�鍂�x�f�f�E���ÃV�X�e���ɎQ�����āF�l�H�m�\��L���铝������f�Îx���V�X�e���̊J��. ��ᇓ��� 27: 120-126, 2021. - Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Ohtani S, Shimizu C, Hashigaki S, Muramatsu Y, Umeyama Y, Iwata H, Toi M.
Analysis of subsequent therapy in Japanese patients with hormone receptor?positive/human epidermal growth factor receptor 2?negative advanced breast cancer who received palbociclib plus endocrine therapy in PALOMA-2 and -3. Breast Cancer 28: 335-345, 2021. - ������.
ASCO 2021 meeting report�F������. ��ᇓ��� 28: 426-431, 2021. - Maeshima Y, Sakai T, Ogiya A, Takahashi Y, Miyagi Y, Kokubu Y, Osako T, Ito Y, Takahashi S, Ohno S, Ueno T.
Assessment of axillary node status by ultrasound after neoadjuvant chemotherapy in patients with clinically node-positive breast cancer according to breast cancer subtype. Sci. Rep. 11: 10858, 2021. - ����R�L��, ���엘��.
Breast and Endocrine Tumor�F���B�������ᇁFAntibody-Drug Conjugate�̗��_�ƗՏ����p�FTNBC�ł̉��p(Sacituzumab Govitecan-Hziy). ���Ɖ��w�Ö@ 48: 1458-1462, 2021. - Ban K, Tsunoda H, Togashi S, Kawaguchi Y, Sato T, Takahashi Y, Nagatsuka Y.
Breast cancer screening using digital breast tomosynthesis compared to digital mammography alone for Japanese women. Breast Cancer 28: 459-464, 2021. - Yokoe T, Kurozumi S, Nozawa K, Ozaki Y, Maeda T, Yazaki S, Onishi M, Fujimoto A, Nakayama S, Tsuboguchi Y, Iwasa T, Sakai H, Ogata M, Terada M, Nishimura M, Onoe T, Masuda J, Kurikawa M, Isaka H, Hagio K, Shimomura A, Okumura Y, Futamura M, Shimokawa M, Takano T.
Clinical benefit of treatment after trastuzumab emtansine for HER2-positive metastatic breast cancer: A real-world multi-centre cohort study in Japan (WJOG12519B). Breast Cancer 28: 581-591, 2021. - Yamamoto M, Yoshida M, Furuse J, Sano K, Ohtsuka M, Yamashita S, Beppu T, Iwashita Y, Wada K, Takako N E, Sakamoto K, Hayano K, Mori Y, Asai K, Matsuyama R, Hirashita T, Hibi T, Sakai N, Tabata T, Kawakami H, Takeda H, Mizukami T, Ozaka M, Ueno M, Naito Y, Okano N, Ueno T, Hijioka S, Shikata S, Ukai T, Strasberg S, Sarr M G, Jagannath P, Hwang T L, Han H S, Yoon Y S, Wang H J, Luo S C, Adam R, Gimenez M, Scatton O, Oh D Y, Takada T.
Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J. Hepatobiliary Pancreat. Sci. 28: 1-25, 2021. - Aogi K, Watanabe K, Kitada M, Sangai T, Ohtani S, Aruga T, Kawagichi H, Fujisawa T, Maeda S, Morimoto T, Sato N, Takao S, Morita S, Masuda N, Toi M, Ohno S.
Clinical usefulness of eribulin as first- or second-line chemotherapy for recurrent HER2-negative breast cancer: A randomized phase II study (JBCRG-19). Int. J. Clin. Oncol. 26: 1229-1236, 2021. - Aogi K, Watanabe K, Kitada M, Sangai T, Ohtani S, Aruga T, Kawagichi H, Fujisawa T, Maeda S, Morimoto T, Sato N, Takao S, Morita S, Masuda N, Toi M, Ohno S.
Correction to: Clinical usefulness of eribulin as first- or second-line chemotherapy for recurrent HER2-negative breast cancer: a randomized phase II study (JBCRG-19). Int. J. Clin. Oncol. 26: 1237, 2021. - Inokuchi M, Kutomi G, Kijima Y, Sakai T, Sawaki M, Shien T, Hanamura N, Yano K, Wada N, Saji S, Iwata H.
Correction to: The Japanese Breast Cancer Society clinical practice guidelines for surgical treatment of breast cancer, 2018 edition. Breast Cancer 28: 989, 2021. - Shimoi T, Nagai S E, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T.
Correction to: The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer 28: 985-986, 2021. - Takashima T, Hara F, Iwamoto T, Uemura Y, Ohsumi S, Yotsumoto D, Hozumi Y, Watanabe T, Saito T, Watanabe K I, Tsurutani J, Toyama T, Akabane H, Nishimura R, Taira N, Ohashi Y, Mukai H.
A correlation analysis between metabolism-related genes and treatment response to S-1 as first-line chemotherapy for metastatic breast cancer: The SELECT BC-EURECA study. Clin. Breast Cancer 21: 450-457, 2021. - Takahashi M, Ohtani S, Nagai S E, Takashima S, Yamaguchi M, Tsuneizumi M, Komoike Y, Osako T, Ito Y, Ikeda M, Ishida K, Nakayama T, Takashima T, Asakawa T, Matsumoto S, Shimizu D, Masuda N.
The efficacy and safety of pertuzumab plus trastuzumab and docetaxel as a first-line therapy in Japanese patients with inoperable or recurrent HER2-positive breast cancer: The COMACHI study. Breast Cancer Res. Treat. 185: 125-134, 2021. - Yamashita T, Kawaguchi H, Masuda N, Kitada M, Narui K, Hattori M, Yoshinami T, Matsunami N, Yanagihara K, Kawasoe T, Nagashima T, Bando H, Yano H, Hasegawa Y, Nakamura R, Kashiwaba M, Morita S, Ohno S, Toi M.
Efficacy of the eribulin, pertuzumab, and trastuzumab combination therapy for human epidermal growth factor receptor 2-positive advanced or metastatic breast cancer: A multicenter, single arm, phase II study (JBCRG-M03 study). Invest. New Drugs. 39: 217-225, 2021. - Masuda N, Bando H, Yamanaka T, Kadoya T, Takahashi M, Nagai S E, Ohtani S, Aruga T, Suzuki E, Kikawa Y, Yasojima H, Kasai H, Ishiguro H, Kawabata H, Morita S, Haga H, Kataoka T R, Uozumi R, Ohno S, Toi M.
Eribulin-based neoadjuvant chemotherapy for triple-negative breast cancer patients stratified by homologous recombination deficiency status: A multicenter randomized phase II clinical trial. Breast Cancer Res. Treat. 188: 117-131, 2021. - Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio I T, Zackrisson S, Senkus E.
Erratum to "Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up": Annals of Oncology 30; 2019: 1194-1220. Ann. Oncol. 32: 284, 2021. - Kokubu Y, Yamada K, Tanabe M, Izumori A, Kato C, Horii R, Ohno S, Matsueda K.
Evaluating the usefulness of breast strain elastography for intraductal lesions. J. Med. Ultrason. 48: 63-70, 2021. - Hagiwara Y, Sawaki M, Uemura Y, Kawahara T, Shimozuma K, Ohashi Y, Takahashi M, Saito T, Baba S, Kobayashi K, Mukai H, Taira N.
Impact of chemotherapy on cognitive functioning in older patients with HER2-positive breast cancer: A sub-study in the RESPECT trial. Breast Cancer Res. Treat. 188: 675-683, 2021. - Inoue K, Masuda N, Iwata H, Takahashi M, Ito Y, Miyoshi Y, Nakayama T, Mukai H, van der Walt J S, Mori J, Sakaguchi S, Kawaguchi T, Tanizawa Y, Llombart-Cussac A, Sledge G W, Jr., Toi M.
Japanese subpopulation analysis of MONARCH 2: Phase 3 study of abemaciclib plus fulvestrant for treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer that progressed on endocrine therapy. Breast Cancer 28: 1038-1050, 2021. - Tokunaga E, Masuda N, Yamamoto N, Iwata H, Bando H, Aruga T, Ohtani S, Fujisawa T, Takano T, Inoue K, Suganuma N, Takada M, Aogi K, Sakurai K, Shigematsu H, Kuroi K, Haga H, Ohno S, Morita S, Toi M.
Long-term outcomes of a randomized study of neoadjuvant induction dual HER2 blockade with trastuzumab and lapatinib followed by weekly paclitaxel plus dual HER2 blockade for HER2-positive primary breast cancer (Neo-Lath Study). Cancers (Basel) 13: 4008, 2021. - Futamura M, Oba M, Masuda N, Bando H, Okada M, Yamamoto Y, Kin T, Saeki T, Nagashima T, Kuwayama T, Toh U, Hirano A, Inokuchi M, Yamagami K, Mizuno Y, Kojima Y, Nakayama T, Yasojima H, Ohno S.
Meta-analysis of nanoparticle albumin-bound paclitaxel used as neoadjuvant chemotherapy for operable breast cancer based on individual patient data (JBCRG-S01 study). Breast Cancer 28: 1023-1037, 2021. - Maeshima Y, Osako T, Morizono H, Yunokawa M, Miyagi Y, Kikuchi M, Ueno T, Ohno S, Akiyama F.
Metastatic ovarian cancer spreading into mammary ducts mimicking an in situ component of primary breast cancer: A case report. J. Med. Case Rep. 15: 78, 2021. - Oyakawa T, Inagaki L, Hua Z, Ebihara A, Takano T, Ohno S, Shiga T.
Myocardial dysfunction caused by abemaciclib: A case report. Int. Cancer Conf. J. 10: 324-328, 2021. - Akiya M, Osako T, Morizono H, Furuta N, Kikuchi M, Ueno T, Ohno S, Takeuchi K.
Myofibroblastoma of the breast showing rare palisaded morphology and uncommon desmin- and CD34-negative immunophenotype: A case report. Pathol. Int. 71: 548-555, 2021. - Ozaki Y, Miura S, Oki R, Morikawa T, Uchino K.
Neuroendocrine neoplasms of the breast: The latest WHO classification and review of the literature. Cancers (Basel) 14: 196, 2021. - Yoshida R, Hagio T, Kaneyasu T, Gotoh O, Osako T, Tanaka N, Amino S, Yaguchi N, Nakashima E, Kitagawa D, Ueno T, Ohno S, Nakajima T, Nakamura S, Miki Y, Hirota T, Takahashi S, Matsuura M, Noda T, Mori S.
Pathogenicity assessment of variants for breast cancer susceptibility genes based on BRCAness of tumor sample. Cancer Sci. 112: 1310-1319, 2021. - Masuda J, Ozaki Y, Hara F, Kitano S, Takano T.
Pembrolizumab plus chemotherapy in triple-negative breast cancer. Lancet 398: 24, 2021. - Fukada I, Ito Y, Kondo N, Ohtani S, Hattori M, Tokunaga E, Matsunami N, Mashino K, Kosaka T, Tanabe M, Yotsumoto D, Yamanouchi K, Sawaki M, Kashiwaba M, Kawabata H, Kuroi K, Morita S, Ohno S, Toi M, Masuda N.
A phase II study of sequential treatment with anthracycline and taxane followed by eribulin in patients with HER2-negative, locally advanced breast cancer (JBCRG-17). Breast Cancer Res. Treat. 190: 425-434, 2021. - Horii R, Nitta H, Nojima M, Maruyama R, Ueno T, Ito Y, Ohno S, Banks P, Kanda H, Akiyama F.
Predictive significance of HER2 intratumoral heterogeneity, determined by simultaneous gene and protein analysis, for resistance to trastuzumab-based treatments for HER2-positive breast cancer. Virchows Arch. 479: 13-21, 2021. - Iwatani T, Hara F, Shien T, Sasaki K, Katayama H, Fukuda H, Shiroiwa T, Iwata H.
Prospective observational study estimating willingness-to-pay for breast cancer treatments through contingent valuation method in Japanese breast cancer patients (JCOG1709A). Jpn. J. Clin. Oncol. 51: 498-503, 2021. - Yamamoto Y, Yamashiro H, Toh U, Kondo N, Nakamura R, Kashiwaba M, Takahashi M, Tsugawa K, Ishikawa T, Nakayama T, Ohtani S, Takano T, Fujisawa T, Toyama T, Kawaguchi H, Mashino K, Tanino Y, Morita S, Toi M, Ohno S.
Prospective observational study of bevacizumab combined with paclitaxel as first- or second-line chemotherapy for locally advanced or metastatic breast cancer: the JBCRG-C05 (B-SHARE) study. Breast Cancer 28: 145-160, 2021. - Ohno S, Saji S, Masuda N, Tsuda H, Akiyama F, Kurosumi M, Shimomura A, Sato N, Takao S, Ohsumi S, Tokuda Y, Inaji H, Watanabe T, Ohashi Y.
Relationships between pathological factors and long-term outcomes in patients enrolled in two prospective randomized controlled trials comparing the efficacy of oral tegafur-uracil with CMF (N·SAS-BC 01 trial and CUBC trial). Breast Cancer Res. Treat. 186: 135-147, 2021. - Ohsumi S, Kiyoto S, Takahashi M, Hara F, Takashima S, Aogi K, Matsuda M, Yamamura N, Doi M.
Scalp cooling for hair loss prevention in female Japanese breast cancer patients receiving (neo)adjuvant chemotherapy. Support. Care Cancer 29: 437-443, 2021. - Chin Y M, Takahashi Y, Chan H T, Otaki M, Fujishima M, Shibayama T, Miki Y, Ueno T, Nakamura Y, Low S K.
Ultradeep targeted sequencing of circulating tumor DNA in plasma of early and advanced breast cancer. Cancer Sci. 112: 454-464, 2021. - Ozaki Y, Masuda J, Kataoka A, Oikado K, Uehiro N, Inagaki L, Kato C, Morizono H, Takano T, Ueno T, Ohno S.
Utility of preoperative computed tomography scans for coronavirus disease in a cancer treatment center. Cancer Cell 39: 9-10, 2021. - Partridge A H, Niman S M, Ruggeri M, Peccatori F A, Azim H A, Jr., Colleoni M, Saura C, Shimizu C, Sætersdal A B, Kroep J R, Mailliez A, Warner E, Borges V F, Amant F, Gombos A, Kataoka A, Rousset-Jablonski C, Borstnar S, Takei J, Lee J E, Walshe J M, Borrego M R, Moore H C, Saunders C, Cardoso F, Susnjar S, Bjelic-Radisic V, Smith K L, Piccart M, Korde L A, Goldhirsch A, Gelber R D, Pagani O.
Who are the women who enrolled in the POSITIVE trial: A global study to support young hormone receptor positive breast cancer survivors desiring pregnancy. Breast 59: 327-338, 2021. - ������.
�z��������e�̗z��HER2�A�������ɂ�����Oncotype DX�̃G�r�f���X�ƓK��. ��ᇓ��� 28: 306-311, 2021. - ������.
�����ɂ�����Ɖu�`�F�b�N�|�C���g�j�Q��̐V���ȓW�J�F�z��������e�̗z��������HER2�z�������ɂ�����Ɖu�`�F�b�N�|�C���g�j�Q�܂̓W�J. �����̗Տ� 36: 173-179, 2021. - �ܖ�����, �ɓ��Ǒ�, ������L, �Ɖ��Ȃ�.
�����p�O���w�Ö@�ɂ��T�u�^�C�v���ω�����1��. Cancer Borad Breast 6: 77-87, 2021. - ������G�q, �x�䗝�G, �{��R��, �����D��, ���}��, ���M�V, ���^�i.
���B��ᇂ̑g�D�w�I���ނ̉����ƐZ�������NJ��̒����g�f�f. Japanese Journal of Medical Ultrasonics 48: 241-247, 2021. - ���V�r��, �㐙��], ���D�M, �ݗm��, �c�ߔE, ���^�i.
��t�̏Љ�挈��v���Ɋւ���A���P�[�g����. ���{��t��G�� 150: 1067-1071, 2021.
2020�N
- Nakajima E, Iwase T, Miyagi Y, Fujita T, Ikeda N, Ishikawa T, Iwata H.
Association of parity and infant feeding method with breast density on mammography. Acad. Radiol. 27: e24-e26, 2020. - Ueno T.
Biomarkers of neoadjuvant/adjuvant endocrine therapy for ER-positive/HER2-negative breast cancer. Chin. Clin. Oncol. 9: 35, 2020. - Fukuoka M, Ueno T, Yoshida K, Kikuchi M, Takeuchi K, Ohno S.
Breast Castleman disease. Breast J. 26: 1855-1856, 2020. - Ohishi Y, Mitsuda A, Ejima K, Morizono H, Yano T, Yokoyama M, Takeuchi K, Fujiwara M, Nemoto T, Minabe T.
Breast implant-associated anaplastic large-cell lymphoma: First case detected in a Japanese breast cancer patient. Breast Cancer 27: 499-504, 2020. - Naito Y, Kai Y, Ishikawa T, Fujita T, Uehara K, Doihara H, Tokunaga S, Shimokawa M, Ito Y, Saeki T.
Chemotherapy-induced nausea and vomiting in patients with breast cancer: a prospective cohort study. Breast Cancer 27: 122-128, 2020. - Shibayama T, Low S K, Ono M, Kobayashi T, Kobayashi K, Fukada I, Ito Y, Ueno T, Ohno S, Nakamura Y, Takahashi S.
Clinical significance of gene mutation in ctDNA analysis for hormone receptor-positive metastatic breast cancer. Breast Cancer Res. Treat. 180: 331-341, 2020. - Inari H, Teruya N, Kishi M, Horii R, Akiyama F, Takahashi S, Ito Y, Ueno T, Iwase T, Ohno S.
Clinicopathological features of breast cancer patients with internal mammary and/or supraclavicular lymph node recurrence without distant metastasis. BMC Cancer 20: 932, 2020. - Tokunaga E, Ijichi H, Tajiri W, Masuda T, Takizawa K, Ueo H, Koga C, Tanaka J, Nakamura Y, Ohno S, Taguchi K, Okamoto M.
The comparison of the anatomic stage and pathological prognostic stage according to the AJCC 8th edition for the prognosis in Japanese breast cancer patients: data from a single institution. Breast Cancer 27: 1137-1146, 2020. - Tsuda H, Tsugawa K, Akiyama F, Horii R, Kurosumi M, Moriya T, Takano T, Takei H, Nakayama T, Miyagi Y, Yamauchi C, Yamashita T, Aogi K, Mukai H, Sugie T, Iwata H, Masuda S.
Correction to: Histological classification of breast tumors in the General Rules for Clinical and Pathological Recording of Breast Cancer (18th edition). Breast Cancer 27: 792, 2020. - Yamashita T, Masuda N, Saji S, Araki K, Ito Y, Takano T, Takahashi M, Tsurutani J, Koizumi K, Kitada M, Kojima Y, Sagara Y, Tada H, Iwasa T, Kadoya T, Iwatani T, Hasegawa H, Morita S, Ohno S.
Correction to: Trastuzumab, pertuzumab, and eribulin mesylate versus trastuzumab, pertuzumab, and a taxane as a first-line or second-line treatment for HER2-positive, locally advanced or metastatic breast cancer: study protocol for a randomized controlled, non-inferiority, phase III trial in Japan (JBCRG-M06/EMERALD). Trials 21: 503, 2020. - Iwata H, Inoue K, Kaneko K, Ito Y, Tsugawa K, Hasegawa A, Nakagawa S, Kuratomi H, Tamura K.
Corrigendum to: Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn. J. Clin. Oncol. 50: 223, 2020. - Okamoto M, Tajiri W, Ueo H, Masuda T, Ijichi H, Koga C, Nakamura Y, Taguchi K, Ohno S, Tokunaga E.
Efficacy of adjuvant combination therapy with trastuzumab and chemotherapy in HER2-positive early breast cancer: A single institutional cohort study from clinical practice. Anticancer Res. 40: 3315-3323, 2020. - O'Shaughnessy J, Cortes J, Twelves C, Goldstein L J, Alexis K, Xie R, Barrios C, Ueno T.
Efficacy of eribulin for metastatic breast cancer based on localization of specific secondary metastases: a post hoc analysis. Sci. Rep. 10: 11203, 2020. - Suzuki R, Yoshida M, Oguchi M, Yoshioka Y, Tokumatsu K, Osako T, Ono S, Ueno T, Miyagi Y.
Efficacy of radiation boost after breast-conserving surgery for breast cancer with focally positive, tumor-exposed margin. J. Radiat. Res. 61: 440-446, 2020. - Takano T.
The era of meta-analyses-based recommendations: how were the Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer established? Breast Cancer 27: 332-333, 2020. - Masuda T, Noda M, Kitagawa A, Hu Q, Fujii A, Ito S, Kosai K, Ando Y, Matsumoto Y, Ohtsu H, Uchida H, Ohno S, Mimori K.
The expression level of PD-L1 (CD274) mRNA in peripheral blood is a potential biomarker for predicting recurrence in breast cancer. Anticancer Res. 40: 3733-3742, 2020. - Kawaguchi H, Masuda N, Nakayama T, Aogi K, Anan K, Ito Y, Ohtani S, Sato N, Saji S, Takano T, Tokunaga E, Nakamura S, Hasegawa Y, Hattori M, Fujisawa T, Morita S, Yamaguchi M, Yamashita H, Yamashita T, Yamamoto Y, Yotsumoto D, Toi M, Ohno S.
Factors associated with prolonged overall survival in patients with postmenopausal estrogen receptor-positive advanced breast cancer using real-world data: a follow-up analysis of the JBCRG-C06 Safari study. Breast Cancer 27: 389-398, 2020. - Shimoi T, Sagara Y, Hara F, Toyama T, Iwata H.
First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer 27: 340-346, 2020. - Yamamoto Y, Ueno T.
Focused issue "Neoadjuvant/adjuvant treatment for early breast cancer". Chin. Clin. Oncol. 9: 26, 2020. - Luangxay T, Osako T, Yonekura R, Sugiura Y, Kikuchi M, Gomi N, Ueno T, Ohno S, Machinami R, Takeuchi K, Akiyama F.
Giant cell tumor of soft tissue of the breast: Case report with H3F3A mutation analysis and review of the literature. Pathol. Res. Pract. 216: 152750, 2020. - �����G��, ���M�V, ���^�i.
HBOC�ɂ�������[�\�h�؏��FRRM�̎��ہF�ی��K�p�ȑO�Ƃ��ꂩ��. �����̗Տ� 35: 339-346, 2020. - Yokokawa T, Suzuki K, Sugisaki T, Kobayashi K, Shouji D, Watanabe H, Kawakami K, Takiguchi T, Aoyama T, Kobayashi K, Takahashi S, Ito Y, Ohno S, Hama T.
Impact of primary pegfilgrastim prophylaxis on relative dose intensity in neoadjuvant/adjuvant FEC-100 chemotherapy. Anticancer Res. 40: 915-921, 2020. - Inokuchi M, Kutomi G, Kijima Y, Sakai T, Sawaki M, Shien T, Hanamura N, Yano K, Wada N, Saji S, Iwata H.
The Japanese Breast Cancer Society clinical practice guidelines for surgical treatment of breast cancer, 2018 edition. Breast Cancer 27: 4-8, 2020. - Shimoi T, Nagai S E, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T.
The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer 27: 322-331, 2020. - Sakai T, Ueno T.
Local management after neoadjuvant treatment for breast cancer. Chin. Clin. Oncol. 9: 34, 2020. - Hirai T, Nemoto A, Ito Y, Matsuura M.
Meta-analyses on progression-free survival as a surrogate endpoint for overall survival in triple-negative breast cancer. Breast Cancer Res. Treat. 181: 189-198, 2020. - Rubio I T, Kontos M, Vrancken-Peeters M, Rouzier R, Skandarajah A R, Galimberti V, Kroman N, Caballero C, Ohno S.
Missed opportunities and challenges for surgical breast cancer research in the era of personalized cancer treatment. Eur. J. Surg. Oncol. 46: 501-503, 2020. - Hosonaga M, Saya H, Arima Y.
Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 39: 711-720, 2020. - Ueno T, Masuda N, Sato N, Ohtani S, Yamamura J, Matsunami N, Kashiwaba M, Takano T, Takahashi M, Kaneko K, Ohno S, Morita S, Toi M.
Multicenter study of primary systemic therapy with docetaxel, cyclophosphamide and trastuzumab for HER2-positive operable breast cancer: the JBCRG-10 study. Jpn. J. Clin. Oncol. 50: 3-11, 2020. - Masuda T, Ueo H, Kai Y, Noda M, Hu Q, Sato K, Fujii A, Hayashi N, Tsuruda Y, Otsu H, Kuroda Y, Eguchi H, Ohno S, Mimori K, Ueo H.
N-cadherin mRNA levels in peripheral blood could be a potential indicator of new metastases in breast cancer: A pilot study. Int. J. Mol. Sci. 21: E511, 2020. - Iwamoto T, Hara F, Uemura Y, Mukai H, Watanabe T, Ohashi Y.
NSAS-BC02 substudy of chemotherapy-induced amenorrhea (CIA) in premenopausal patients who received either taxane alone or doxorubicin(A) cyclophosphamide(C) followed by taxane as postoperative chemotherapy. Breast Cancer Res. Treat. 182: 325-332, 2020. - Yamashiro H, Iwata H, Masuda N, Yamamoto N, Nishimura R, Ohtani S, Sato N, Takahashi M, Kamio T, Yamazaki K, Saito T, Kato M, Lee T, Kuroi K, Takano T, Yasuno S, Morita S, Ohno S, Toi M.
Outcomes of trastuzumab therapy in HER2-positive early breast cancer patients: extended follow-up of JBCRG-cohort study 01. Breast Cancer 27: 631-641, 2020. - Tsuda M, Ishiguro H, Toriguchi N, Masuda N, Bando H, Ohgami M, Homma M, Morita S, Yamamoto N, Kuroi K, Yanagita Y, Takano T, Shimizu S, Toi M.
Overnight fasting before lapatinib administration to breast cancer patients leads to reduced toxicity compared with nighttime dosing: a retrospective cohort study from a randomized clinical trial. Cancer Med. 9: 9246-9255, 2020. - Horii R, Tsuda H, Masuda S, Sugita H, Togashi K, Ohno S, Akiyama F.
Performance analysis of the anti-Ki67 antibody clone 30-9 for immunohistochemical staining of breast cancer. Breast Cancer 27: 1058-1064, 2020. - Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T, Nakanishi Y, Nakayama K I, Ohno S, Mimori K.
Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 111: 924-931, 2020. - Bando H, Kataoka A, Tamaki K, Kobayashi M, Tamura N, Ozawa M, Kawano J, Fukatsu Y, Kitano A, Shiota K, Yamauchi H.
Physician's knowledge, attitudes and practice pattern for breast cancer diagnosed during pregnancy: a survey among breast care specialists in Japan. Breast Cancer 27: 796-802, 2020. - Kaneyasu T, Mori S, Yamauchi H, Ohsumi S, Ohno S, Aoki D, Baba S, Kawano J, Miki Y, Matsumoto N, Nagasaki M, Yoshida R, Akashi-Tanaka S, Iwase T, Kitagawa D, Masuda K, Hirasawa A, Arai M, Takei J, Ide Y, Gotoh O, Yaguchi N, Nishi M, Kaneko K, Matsuyama Y, Okawa M, Suzuki M, Nezu A, Yokoyama S, Amino S, Inuzuka M, Noda T, Nakamura S.
Prevalence of disease-causing genes in Japanese patients with BRCA1/2-wildtype hereditary breast and ovarian cancer syndrome. NPJ Breast Cancer 6: 25, 2020. - Sawaki M, Taira N, Uemura Y, Saito T, Baba S, Kobayashi K, Kawashima H, Tsuneizumi M, Sagawa N, Bando H, Takahashi M, Yamaguchi M, Takashima T, Nakayama T, Kashiwaba M, Mizuno T, Yamamoto Y, Iwata H, Kawahara T, Ohashi Y, Mukai H.
Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J. Clin. Oncol. 38: 3743-3752, 2020. - Tsurutani J, Hara F, Kitada M, Takahashi M, Kikawa Y, Kato H, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Taira N, Takashima T, Kashiwabara K, Aihara T, Mukai H.
Randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast 55: 63-68, 2020. - Ishiguro H, Masuda N, Sato N, Higaki K, Morimoto T, Yanagita Y, Mizutani M, Ohtani S, Kaneko K, Fujisawa T, Takahashi M, Kadoya T, Matsunami N, Yamamoto Y, Ohno S, Takano T, Morita S, Tanaka-Mizuno S, Toi M.
A randomized study comparing docetaxel/cyclophosphamide (TC), 5-fluorouracil/epirubicin/cyclophosphamide (FEC) followed by TC, and TC followed by FEC for patients with hormone receptor-positive HER2-negative primary breast cancer. Breast Cancer Res. Treat. 180: 715-724, 2020. - Masuda N, Ohtani S, Takano T, Inoue K, Suzuki E, Nakamura R, Bando H, Ito Y, Ishida K, Yamanaka T, Kuroi K, Yasojima H, Kasai H, Takasuka T, Sakurai T, Kataoka T R, Morita S, Ohno S, Toi M.
A randomized, 3-arm, neoadjuvant, phase 2 study comparing docetaxel + carboplatin + trastuzumab + pertuzumab (TCbHP), TCbHP followed by trastuzumab emtansine and pertuzumab (T-DM1+P), and T-DM1+P in HER2-positive primary breast cancer. Breast Cancer Res. Treat. 180: 135-146, 2020. - Lin C H, Yap Y S, Lee K H, Yeo W, Ueno T, Li H, Huang S M, Lu Y S.
Response to Sung, Rosenberg, and Yang. J. Natl. Cancer Inst. 112: 547-548, 2020. - Yap Y S, Chiu J, Ito Y, Ishikawa T, Aruga T, Kim S J, Toyama T, Saeki T, Saito M, Gounaris I, Su F, Ji Y, Han Y, Gazdoiu M, Masuda N.
Ribociclib, a CDK 4/6 inhibitor, plus endocrine therapy in Asian women with advanced breast cancer. Cancer Sci. 111: 3313-3326, 2020. - Fujiwara Y, Sato Y, Wang X, Oikado K, Sato Y, Fukuda N, Enokida T, Takeda K, Ohkushi D, Hayama B, Egi Y, Tokai Y, Yamada Y, Nakajima Y, Kubota M, Haruki S, Shimizu T, Uchida Y, Utsugi K, Ito Y, Ohno S, Takahashi S, Tsuchida T.
Screening for COVID-19 in symptomatic cancer patients in a cancer hospital. Cancer Cell 38: 609-610, 2020. - Inari H, Teruya N, Kishi M, Horii R, Akiyama F, Takahashi S, Ito Y, Ueno T, Iwase T, Ohno S.
Survival in cytologically proven node-positive breast cancer patients with nodal pathological complete response after neoadjuvant chemotherapy. Cancers (Basel) 12: E2633, 2020. - Modi S, Saura C, Yamashita T, Park Y H, Kim S B, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im S A, Lee K S, Hurvitz S A, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I.
Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382: 610-621, 2020. - Yamashita T, Masuda N, Saji S, Araki K, Ito Y, Takano T, Takahashi M, Tsurutani J, Koizumi K, Kitada M, Kojima Y, Sagara Y, Tada H, Iwasa T, Kadoya T, Iwatani T, Hasegawa H, Morita S, Ohno S.
Trastuzumab, pertuzumab, and eribulin mesylate versus trastuzumab, pertuzumab, and a taxane as a first-line or second-line treatment for HER2-positive, locally advanced or metastatic breast cancer: study protocol for a randomized controlled, non-inferiority, phase III trial in Japan (JBCRG-M06/EMERALD). Trials 21: 391, 2020. - Izumori A, Kokubu Y, Sato K, Gomi N, Morizono H, Sakai T, Horii R, Akiyama F, Iwase T, Ohno S.
Usefulness of second-look ultrasonography using anatomical breast structures as indicators for magnetic resonance imaging-detected breast abnormalities. Breast Cancer 27: 129-139, 2020. - ������, ���^�i.
�Q�m����Âƕ��q�W�I���Â̍ŐV���F���q�W�I���Â̎���(����)�F������. ��ଂƌ��� 97: 402-406, 2020. - ���c�^�ޔ�, ���, �Љ�����, �x�䗝�G, ���M�V, �H�R��, ���^�i.
���[�����؏���11�N�œ�����Paget�^�Ĕ��ƑΑ����|�����p�ߓ]�ڂ�����������Z�������NJ���1��. �����̗Տ� 35: 131-135, 2020. - �F�����, ���o��, �R��, ��،���, �ɓ��Ǒ�, �_�q�O.
�������p�����w�Ö@�Ő��f��Ƃ��Ďg�p����I�����U�s���̌����l�ɗ^����e��. ���Ɖ��w�Ö@ 47: 1471-1475, 2020. - ���^�i.
���{�̓�����Տ������E�V���ÊJ���̌���ƓW�]�FJBCRG�̌���ƓW�]. ��ᇓ��� 26: 123-130, 2020. - ����R�L��, ���엘��.
���{�̓�����Տ������E�V���ÊJ���̌���ƓW�]�F������ɑ���V�������Ö@�̊J���F�Ɖu�`�F�b�N�|�C���g�j�Q��. ��ᇓ��� 26: 147-156, 2020. - �X���p�q, ���^�i.
���E��ᇊw�F���[�C���v�����g�֘A��������זE�^�����p��(BIA-ALCL). ��w�̂���� 273: 660-661, 2020. - �[�c�ꕽ.
���]�ڂ������̗Տ�. �Տ��摜 36: 911-917, 2020.
2019�N
- Sato A, Sakai T, Iwase T, Kano F, Kimura K, Ogiya A, Koizumi M, Tanabe M, Horii R, Akiyama F, Ueno T, Ohno S.
Altered lymphatic drainage patterns in re-operative sentinel lymph node biopsy for ipsilateral breast tumor recurrence. Radiat. Oncol. 14: 159, 2019. - Miyoshi Y, Shien T, Ogiya A, Ishida N, Yamazaki K, Horii R, Horimoto Y, Masuda N, Yasojima H, Inao T, Osako T, Takahashi M, Tomioka N, Wanifuchi-Endo Y, Hosoda M, Doihara H, Yamashita H.
Associations in tumor infiltrating lymphocytes between clinicopathological factors and clinical outcomes in estrogen receptor-positive/human epidermal growth factor receptor type 2 negative breast cancer. Oncol. Lett. 17: 2177-2186, 2019. - Ueno T, Saji S, Masuda N, Iwata H, Kuroi K, Sato N, Takei H, Yamamoto Y, Ohno S, Yamashita H, Hisamatsu K, Aogi K, Sasano H, Toi M.
Changes in Recurrence Score by neoadjuvant endocrine therapy of breast cancer and their prognostic implication. ESMO Open 4: e000476, 2019. - Honma N, Makita M, Saji S, Mikami T, Ogata H, Horii R, Akiyama F, Iwase T, Ohno S.
Characteristics of adverse events of endocrine therapies among older patients with breast cancer. Support. Care Cancer 27: 3813-3822, 2019. - Rothe F, Silva M J, Venet D, Campbell C, Bradburry I, Rouas G, de Azambuja E, Maetens M, Fumagalli D, Rodrik-Outmezguine V, Di Cosimo S, Rosa D, Chia S, Wardley A, Ueno T, Janni W, Huober J, Baselga J, Piccart M, Loi S, Sotiriou C, Dawson S J, Ignatiadis M.
Circulating tumor DNA in HER2-amplified breast cancer: A translational research substudy of the neoALTTO phase III trial. Clin. Cancer Res. 25: 3581-3588, 2019. - Lin C H, Yap Y S, Lee K H, Im S A, Naito Y, Yeo W, Ueno T, Kwong A, Li H, Huang S M, Leung R, Han W, Tan B, Hu F C, Huang C S, Cheng A L, Lu Y S.
Contrasting epidemiology and clinicopathology of female breast cancer in Asians versus the US population. J. Natl. Cancer Inst. 111: 1298-1306, 2019. - Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Mori Y, Hashigaki S, Muramatsu Y, Umeyama Y, Iwata H, Toi M.
Correction to: Neutropenia management with palbociclib in Japanese patients with advanced breast cancer. Breast Cancer 26: 651, 2019. - Yonekura R, Horii R, Iwase T, Gomi N, Kitagawa M, Akiyama F, Ohno S.
A diagnostic strategy for breast calcifications based on a long-term follow-up of 615 lesions. Jpn. J. Radiol. 37: 237-244, 2019. - Ueno T, Masuda N, Kamigaki S, Morimoto T, Saji S, Imoto S, Sasano H, Toi M.
Differential involvement of autophagy and apoptosis in response to chemoendocrine and endocrine therapy in breast cancer: JBCRG-07TR. Int. J. Mol. Sci. 20: Pii:E984, 2019. - Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio I T, Zackrisson S, Senkus E.
Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30: 1194-1220, 2019. - Shimada S, Yoshida R, Nakashima E, Kitagawa D, Gomi N, Horii R, Takeuchi S, Ashihara Y, Kita M, Akiyama F, Ohno S, Saito M, Arai M.
Five screening-detected breast cancer cases in initially disease-free BRCA1 or BRCA2 mutation carriers. Breast Cancer 26: 846-851, 2019. - Robertson J F R, Jiang Z, Di Leo A, Ohno S, Pritchard K I, Ellis M, Bradbury I, Campbell C.
A meta-analysis of clinical benefit rates for fulvestrant 500 mg vs. alternative endocrine therapies for hormone receptor-positive advanced breast cancer. Breast Cancer 26: 703-711, 2019. - Sakai T, Ozkurt E, DeSantis S, Wong S M, Rosenbaum L, Zheng H, Golshan M.
National trends of synchronous bilateral breast cancer incidence in the United States. Breast Cancer Res. Treat. 178: 161-167, 2019. - Sato N, Masuda N, Morimoto T, Ueno T, Kanbayashi C, Kaneko K, Yasojima H, Saji S, Sasano H, Morita S, Ohno S, Toi M.
Neoadjuvant exemestane or exemestane plus docetaxel and cyclophosphamide tailored by clinicopathological response to 12 weeks' exemestane exposure in patients with estrogen receptor-positive breast cancer: A multicenter, open-label, phase II study. Cancer Med. 8: 5468-5481, 2019. - Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Mori Y, Hashigaki S, Muramatsu Y, Umeyama Y, Iwata H, Toi M.
Neutropenia management with palbociclib in Japanese patients with advanced breast cancer. Breast Cancer 26: 637-650, 2019. - Masuda N, Inoue K, Nakamura R, Rai Y, Mukai H, Ohno S, Hara F, Mori Y, Hashigaki S, Muramatsu Y, Nagasawa T, Umeyama Y, Huang X, Iwata H.
Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int. J. Clin. Oncol. 24: 262-273, 2019. - Mukai H, Shimizu C, Masuda N, Ohtani S, Ohno S, Takahashi M, Yamamoto Y, Nishimura R, Sato N, Ohsumi S, Iwata H, Mori Y, Hashigaki S, Muramatsu Y, Nagasawa T, Umeyama Y, Lu D R, Toi M.
Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int. J. Clin. Oncol. 24: 274-287, 2019. - Takahashi M, Masuda N, Nishimura R, Inoue K, Ohno S, Iwata H, Hashigaki S, Muramatsu Y, Umeyama Y, Toi M.
Palbociclib-letrozole as first-line treatment for advanced breast cancer: Updated results from a Japanese phase 2 study. Cancer Med. 9: 4929-4940, 2019. - Ohno S, Mukai H, Narui K, Hozumi Y, Miyoshi Y, Yoshino H, Doihara H, Suto A, Tamura M, Morimoto T, Zaha H, Chishima T, Nishimura R, Ishikawa T, Uemura Y, Ohashi Y.
Participants in a randomized controlled trial had longer overall survival than non-participants: a prospective cohort study. Breast Cancer Res. Treat. 176: 631-635, 2019. - Ishiguro H, Ohno S, Yamamoto Y, Takao S, Sato N, Fujisawa T, Kadoya T, Kuroi K, Bando H, Teramura Y, Iwata H, Tanaka S, Toi M.
Pharmacogenomic-pharmacokinetic study of selective estrogen-receptor modulators with intra-patient dose escalation in breast cancer. Breast Cancer 26: 535-543, 2019. - Yonekura R, Osako T, Iwase T, Ogiya A, Ueno T, Kitagawa M, Ohno S, Akiyama F.
Prognostic impact and possible pathogenesis of lymph node metastasis in ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 174: 103-111, 2019. - Ito H, Ueno T, Suga H, Shiraishi T, Isaka H, Imi K, Miyamoto K, Tada M, Ishizaka Y, Imoto S.
Risk factors for skin flap necrosis in breast cancer patients treated with mastectomy followed by immediate breast reconstruction. World J. Surg. 43: 846-852, 2019. - Iwata H, Inoue K, Kaneko K, Ito Y, Tsugawa K, Hasegawa A, Nakagawa S, Kuratomi H, Tamura K.
Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn. J. Clin. Oncol. 49: 1083-1091, 2019. - Ozkurt E, Sakai T, Wong S M, Tukenmez M, Golshan M.
Survival outcomes for patients with clinical complete response after neoadjuvant chemotherapy: Is omitting surgery an option? Ann. Surg. Oncol. 26: 3260-3268, 2019. - �ܖ�����, �ɓ��Ǒ�, ������L, �Ɖ��Ȃ�.
TIL����HER2�z�������ɏp�O���w�Ö@���{�s��pCR������ꂽ2�Ǘ�. Cancer Borad Breast 5: 5-17, 2019. - Yeo W, Ueno T, Lin C H, Liu Q, Lee K H, Leung R, Naito Y, Park Y H, Im S A, Li H, Yap Y S, Lu Y S.
Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Breast Cancer Res. Treat. 177: 549-559, 2019. - Sagawa N, Ohno S, Hiratsuka T, Kondo N, Iwata H, Bando H, Ohyama T, Ishida M, Kono Y, Nakajima K, Empuku S, Nishikawa S, Irie Y, Inomata M, Kitano S.
The utility of DHL-HisZnNa, a novel antioxidant, against anticancer agent-induced alopecia in breast cancer patients: a multicenter phase II clinical trial. Breast Cancer Res. Treat. 176: 625-630, 2019. - Iwata H, Masuda N, Yamamoto Y, Fujisawa T, Toyama T, Kashiwaba M, Ohtani S, Taira N, Sakai T, Hasegawa Y, Nakamura R, Akabane H, Shibahara Y, Sasano H, Yamaguchi T, Sakamaki K, Bailey H, Cherbavaz D B, Jakubowski D M, Sugiyama N, Chao C, Ohashi Y.
Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res. Treat. 173: 123-133, 2019. - �[�c�ꕽ.
�����`�q�p�l�������̌���ƓW�]�F����Q�m����Ð��i�Ɍ����������J���Ȃ̎�g��. �����̗Տ� 34: 373-384, 2019. - ���M�V.
���ꂾ���͉������Ă����������w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g2019�E2020�N��:���q�W�I��FHER�F�g���X�c�Y�}�u�G���^���V��. YORi-SOU����i�[�V���O 46-47, 2019. - ���M�V.
���ꂾ���͉������Ă����������w�Ö@�̖�F�R����܁E�z�������܁E���q�W�I��E�Ɖu�`�F�b�N�|�C���g�j�Q��E�x���Ö@��F�͂⒲�׃m�[�g2019�E2020�N��:���q�W�I��FHER�F�y���c�Y�}�u(��`�q�g����). YORi-SOU����i�[�V���O 48-49, 2019. - ���ѐS.
������E�w�l�Ȃ���̐V�����W�����ÁFHER2�z����������p�����Â̓W�]. ��ᇓ��� 24: 108-113, 2019. - �k���.
�����J�����g�g�s�b�N�X�FHBOC�̏������Âɂ����čl�����ׂ��|�C���g. Cancer Borad Breast 5: 118-120, 2019. - �����m�q.
�����̐f�f�Ǝ��ÁF�f�f�F���� DCIS�̉摜�f�f�F�}�����O���t�B. �Տ����ː� 64: 423-430, 2019. - �����D��, �x�䗝�G, ������G�q, �x�~�ۍs, �{��R��, ���}��.
�����̐f�f�Ǝ��ÁF�f�f�F�����ʊӕʐf�f ��ᎁF�����g����. �Տ����ː� 64: 353-361, 2019. - ���^�i.
�����f�Â̌���. Medical Science Digest 45: 449-451, 2019. - �ܖ�����, �ɓ��Ǒ�, ������L, �{��R��.
�RHER2�Ö@�ɒ�R����������HER2�z������. Cancer Borad Breast 5: 77-88, 2019. - �A�O�ޒÌb.
����ƃh���[���̈ʒu�W&Dr�̎��_���܂�킩��F����ŁI�h���[���Ǘ��̕K�{�m���@�Ō�̂��߂̃h���[���A�g���X�F(Part2) �ʒu�ʃA�g���X�F�O�����牺(������p��). �i�[�V���O 39: 15, 2019. - ������.
����f�B�x�[�g�Fn0 high-risk HER2�z�������̏p�㎡�ÂŃg���X�c�Y�}�u�̓��^��1�N�K�v���F�u�K�v�ł���v�Ƃ��闧�ꂩ��. Cancer Borad Breast 5: 106-111, 2019. - ���ѐS.
��E�ǗႩ��w�ԁF����Ö@�F�W�����Â̎��H�I(��3��)�x���Ö@�E�ɘa��ÁF�S���Ǐ�Q(�I���R�J���f�B�I���W�[). �� 61: 1868-1871, 2019.


